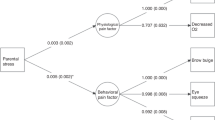
Abstract
Background
The aim of this study was to investigate the influence of early-life pain/stress and medical characteristics on neurobehavioral outcomes in preterm infants.
Methods
A prospective cohort study was conducted with 92 preterm infants (28–32 weeks gestational age [GA]). Early-life pain/stress was measured via the Neonatal Infant Stressor Scale (NISS) during the first 28 days of NICU hospitalization. Neurobehavioral outcomes were evaluated using the NICU Network Neurobehavioral Scale at 36–38 weeks post-menstrual age. Functional regression and machine learning models were performed to investigate the predictors of neurobehavioral outcomes.
Results
Infants experienced daily acute pain/stress (24.99 ± 7.13 frequencies) and chronic events (41.13 ± 17.81 h). Up to 12 days after birth, both higher acute and chronic NISS scores were associated with higher stress scores; and higher chronic NISS scores were also related to lower self-regulation and quality of movement. Younger GA predicted worse neurobehavioral outcomes; GA < 31.57 weeks predicted worse stress/abstinence, self-regulation, and excitability; GA < 30.57 weeks predicted poor quality of movement. A higher proportion of maternal breastmilk intake predicted better self-regulation, excitability, and quality of movement in older GA infants.
Conclusions
Preterm infants are vulnerable to the impact of early-life pain/stress. Neurobehavioral outcomes are positively associated with increased GA and higher maternal breastmilk intake.
Impact
-
During the first 12 days of life, preterm infant neurobehavioral outcomes were vulnerable to the negative impact of acute and chronic pain/stress. Future research is warranted to investigate the long-term effects of early-life pain/stress on neurobehavioral outcomes.
-
Gestational age remains one of the critical factors to predict neurobehavioral outcomes in preterm infants; older gestational age significantly predicted better neurobehavioral outcomes.
-
Feeding with a higher proportion of maternal breastmilk predicted better neurobehavioral outcomes. Future research is warranted to investigate how maternal breastmilk may buffer the negative effects of early-life pain/stress on neurobehavioral outcomes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Field, D. J., Dorling, J. S., Manktelow, B. N. & Draper, E. S. Survival of extremely premature babies in a geographically defined population: prospective cohort study of 1994-9 compared with 2000-5. BMJ 336, 1221–1223 (2008).
Itabashi, K. et al. Mortality rates for extremely low birth weight infants born in Japan in 2005. Pediatrics 123, 445–450 (2009).
Patel, R. M. et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N. Engl. J. Med. 372, 331–340 (2015).
Norman, M. et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA 321, 1188–1199 (2019).
Mooney-Leber, S. M. & Brummelte, S. Neonatal pain and reduced maternal care: early-life stressors interacting to impact brain and behavioral development. Neuroscience 342, 21–36 (2017).
Cong, X. et al. The impact of cumulative pain/stress on neurobehavioral development of preterm infants in the NICU. Early Hum. Dev. 108, 9–16 (2017).
Chau, C. M., Cepeda, I. L., Devlin, A. M., Weinberg, J. & Grunau, R. E. The Val66Met brain-derived neurotrophic factor gene variant interacts with early pain exposure to predict cortisol dysregulation in 7-year-old children born very preterm: Implications for cognition. Neuroscience 342, 188–199 (2017).
Badr, L. K., Abdallah, B. & Kahale, L. A meta-analysis of preterm infant massage: an ancient practice with contemporary applications. MCN Am. J. Matern. Child Nurs. 40, 344–358 (2015).
Ganguly, A., Bhadesia, P. J., Phatak, A. G., Nimbalkar, A. S. & Nimbalkar, S. M. Pain profile of premature infants during routine procedures in neonatal intensive care: an observational study. J. Fam. Med. Prim. Care. 9, 1517–1521 (2020).
Mooney-Leber, S. M. & Brummelte, S. Neonatal pain and reduced maternal care alter adult behavior and hypothalamic-pituitary-adrenal axis reactivity in a sex-specific manner. Dev. Psychobiol. 62, 631–643 (2020).
Zimmerman, K. O. et al. Sedation, analgesia, and paralysis during mechanical ventilation of premature infants. J. Pediatr. 180, 99.e1–104.e1 (2017).
McPherson, C., Miller, S. P., El-Dib, M., Massaro, A. N. & Inder, T. E. The influence of pain, agitation, and their management on the immature brain. Pediatr. Res. 88, 168–175 (2020).
Barrero-Castillero, A., Morton, S. U., Nelson, C. A. 3rd & Smith, V. C. Psychosocial stress and adversity: effects from the perinatal period to adulthood. Neoreviews 20, e686–e696 (2019).
Brummelte, S. et al. Procedural pain and brain development in premature newborns. Ann. Neurol. 71, 385–396 (2012).
Vinall, J. et al. Neonatal pain in relation to postnatal growth in infants born very preterm. Pain 153, 1374–1381 (2012).
Smith, G. C. et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann. Neurol. 70, 541–549 (2011).
Slater, R. et al. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. Neuroimage 52, 583–589 (2010).
Kostovic, I. & Rakic, P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J. Comp. Neurol. 297, 441–470 (1990).
Perry, M. et al. Neonatal pain: perceptions and current practice. Crit. Care Nurs. Clin. North Am. 30, 549–561 (2018).
Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine. Prevention and management of procedural pain in the neonate: an update. Pediatrics 137, e20154271 (2016).
Campbell-Yeo, M. et al. Sustained efficacy of kangaroo care for repeated painful procedures over neonatal intensive care unit hospitalization: a single-blind randomized controlled trial. Pain 160, 2580–2588 (2019).
Banga, S., Datta, V., Rehan, H. S. & Bhakhri, B. K. Effect of sucrose analgesia, for repeated painful procedures, on short-term neurobehavioral outcome of preterm neonates: a randomized controlled trial. J. Trop. Pediatr. 62, 101–106 (2016).
Meesters, N. J. et al. Acute pain assessment in prematurely born infants below 29 weeks: a long way to go. Clin. J. Pain 35, 975–982 (2019).
Wang, Y., Zhao, T., Zhang, Y., Li, S. & Cong, X. Positive effects of kangaroo mother care on long-term breastfeeding rates, growth, and neurodevelopment in preterm infants. Breastfeed. Med. 16, 282–291 (2021).
El-Farrash, R. A. et al. Longer duration of kangaroo care improves neurobehavioral performance and feeding in preterm infants: a randomized controlled trial. Pediatr. Res. 87, 683–688 (2020).
Cheong, J., Burnett, A. C., Treyvaud, K. & Spittle, A. J. Early environment and long-term outcomes of preterm infants. J. Neural Transm. 127, 1–8 (2020).
Bergman, N. J. Birth practices: maternal-neonate separation as a source of toxic stress. Birth Defects Res. 111, 1087–1109 (2019).
Zwicker, J. G. et al. Score for neonatal acute physiology-II and neonatal pain predict corticospinal tract development in premature newborns. Pediatr. Neurol. 48, 123.e1–129.e1 (2013).
Ranger, M. et al. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS ONE 8, e76702 (2013).
Ranger, M. et al. Neonatal pain and infection relate to smaller cerebellum in very preterm children at school age. J. Pediatr. 167, 292–298.e291 (2015).
Grunau, R. E. et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain 143, 138–146 (2009).
Moore, G. A., Cohn, J. F. & Campbell, S. B. Infant affective responses to mother’s still face at 6 months differentially predict externalizing and internalizing behaviors at 18 months. Dev. Psychol. 37, 706–714 (2001).
Ranger, M., Synnes, A. R., Vinall, J. & Grunau, R. E. Internalizing behaviours in school-age children born very preterm are predicted by neonatal pain and morphine exposure. Eur. J. Pain 18, 844–852 (2014).
Newnham, C. A., Inder, T. E. & Milgrom, J. Measuring preterm cumulative stressors within the NICU: the Neonatal Infant Stressor Scale. Early Hum. Dev. 85, 549–555 (2009).
Gao, H. et al. Efficacy and safety of repeated oral sucrose for repeated procedural pain in neonates: a systematic review. Int. J. Nurs. Stud. 62, 118–125 (2016).
Stokowski, L. A. Quantifying neonatal stress in the NICU. Adv. Neonatal Care 9, 205 (2009).
Lester, B. M., Andreozzi-Fontaine, L., Tronick, E. & Bigsby, R. Assessment and evaluation of the high risk neonate: the NICU Network Neurobehavioral Scale. J. Vis. Exp. 3368 (2014).
Lester, B. M., Tronick, E. Z. & Brazelton, T. B. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics 113, 641–667 (2004).
Harris, P. A. et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inform. 95, 103208 (2019).
R Core Team. R: A Language and Environment for Statistical Computing (Computer Program) (R Foundation for Statistical Computing, 2017).
Brockhaus, S., Rügamer, D. & Greven, S. Boosting functional regression models with FDboost. J. Stat. Softw. 94, 1–50 (2020).
Hothorn, T., Hornik, K. & Zeileis, A. Unbiased recursive partitioning: a conditional inference framework. J. Comput. Graph. Stat. 15, 651–674 (2006).
Dutt, S., Sivaraman, A., Savoy, F. & Rajalakshmi, R. Insights into the growing popularity of artificial intelligence in ophthalmology. Indian J. Ophthalmol. 68, 1339–1346 (2020).
Allinson, L. G. et al. Physiological stress responses in infants at 29-32 weeks’ postmenstrual age during clustered nursing cares and standardised neurobehavioural assessments. BMJ Paediatr. Open 1, e000025 (2017).
Tronick, E. Z. et al. Normative neurobehavioral performance of healthy infants on the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics 113, 676–678 (2004).
D’Agata, A. L. et al. FKBP5 genotype and early life stress exposure predict neurobehavioral outcomes for preterm infants. Dev. Psychobiol. 59, 410–418 (2017).
Rantakari, K. et al. Early oxygen levels contribute to brain injury in extremely preterm infants. Pediatr Res. 90, 131–139 (2021).
Nist, M. D., Pickler, R. H., Steward, D. K., Harrison, T. M. & Shoben, A. B. Inflammatory mediators of stress exposure and neurodevelopment in very preterm infants: protocol for the stress neuro-immune study. J. Adv. Nurs. 75, 2236–2245 (2019).
DeMaster, D. et al. Nurturing the preterm infant brain: leveraging neuroplasticity to improve neurobehavioral outcomes. Pediatr. Res. 85, 166–175 (2019).
Rice, D. & Barone, S. Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 108(Suppl. 3), 511–533 (2000).
Wallois, F., Routier, L. & Bourel-Ponchel, E. Impact of prematurity on neurodevelopment. Handb. Clin. Neurol. 173, 341–375 (2020).
Apkarian, A. V., Bushnell, M. C., Treede, R. D. & Zubieta, J. K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 9, 463–484 (2005).
Holsti, L., MacLean, K., Oberlander, T., Synnes, A. & Brant, R. Calmer: a robot for managing acute pain effectively in preterm infants in the neonatal intensive care unit. Pain Rep. 4, e727 (2019).
Pierrat, V. et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ 358, j3448 (2017).
Eeles, A. L. et al. Continuum of neurobehaviour and its associations with brain MRI in infants born preterm. BMJ Paediatr. Open. 1, e000136 (2017).
Brown Belfort, M. The science of breastfeeding and brain development. Breastfeed. Med. 12, 459–461 (2017).
Lechner, B. E. & Vohr, B. R. Neurodevelopmental outcomes of preterm infants fed human milk: a systematic review. Clin. Perinatol. 44, 69–83 (2017).
Pineda, R. et al. Maternal milk and relationships to early neurobehavioral outcome in preterm infants. J. Perinat. Neonatal Nurs. 34, 72–79 (2020).
O’Connor, D. L. et al. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: a randomized clinical trial. JAMA 316, 1897–1905 (2016).
Miller, J. et al. A systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients 10, 707 (2018).
Quigley, M., Embleton, N. D. & McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 6, CD002971 (2018).
Acknowledgements
The authors thank medical and nursing staff in the NICUs of Connecticut Children’s Medical Center at Hartford and Farmington, CT for their support and assistance. We thank Victoria Vazquez, Shari Galvin, Megan Fitzsimons, and Dorothy Vittner for their assistance in recruiting subjects and data collection.
Funding
This study was supported by the NIH/NINR (grant numbers: K23NR014674 and NR016928; PI: X.C.; F31NR019940, PI: T.Z.).
Author information
Authors and Affiliations
Contributions
All authors met the Pediatric Research authorship requirements. Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: all authors. Drafting and revising the manuscript: T.Z., T.G., Y.Z., H.L., and X.C. Thoroughly reviewed and critiqued the manuscript for revision: B.L. and N.H. Final approval of the version to be published: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Patient consent was required for our paper. Eligible infants were identified by the research study personnel after birth and/or admission to the NICU and consent from parents of the eligible infant was required and obtained for participating in our study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, T., Griffith, T., Zhang, Y. et al. Early-life factors associated with neurobehavioral outcomes in preterm infants during NICU hospitalization. Pediatr Res 92, 1695–1704 (2022). https://doi.org/10.1038/s41390-022-02021-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02021-y
This article is cited by
-
Barriers to the implementation of the Newborn Individualized Developmental Care and Assessment Program in neonatal intensive care units and the proposed solutions to improve its implementation: a scoping review
BMC Pediatrics (2025)
-
Behavioral and physiological pain structures of PIPP-R and parental stress: structural equation modeling approach
Pediatric Research (2025)
-
NeoVault: empowering neonatal research through a neonate data hub
BMC Pediatrics (2024)