Abstract
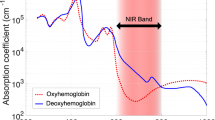
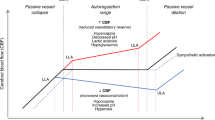
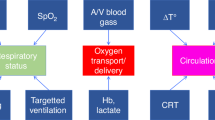
This narrative review focuses on the clinical use and relevance of cerebral oxygenation measured by NIRS during fetal to neonatal transition. Cerebral NIRS(cNIRS) offers the possibility of non-invasive, continuous, and objective brain monitoring in addition to the recommended routine monitoring. During the last decade, with growing interest in early and sensitive brain monitoring, many research groups worldwide have been working with cNIRS and verified the feasibility of cNIRS monitoring immediately after birth. Cerebral hypoxia during fetal to neonatal transition, defined as cerebral oxygenation values below10th percentile, seems to have an impact on neurological outcomes. Feasibility to guide clinical support using cNIRS to reduce the burden of cerebral hypoxia has been shown. It is well known that in some cases cerebral oxygenation follows different patterns than SpO2. Cerebral oxygenation does not only depend on systemic oxygenation, hemoglobin content and cerebral blood flow, but also on cardiocirculatory condition, ventilation, and metabolic parameters. Hence, measurement of cerebral oxygenation may uncover problems not detectable by standard monitoring. Therefore, applying NIRS can provide caregivers a more complete clinical overview, especially in critically ill neonates. In this review, we aim to describe the additional information which can be provided by cNIRS during fetal to neonatal transition.
Impact
-
This narrative review focuses on the clinical use and relevance of cerebral oxygenation measured by near infrared spectroscopy (NIRS) during fetal to neonatal transition.
-
During the last decade, interest on brain monitoring is growing continuously as the measurement of cerebral oxygenation may uncover problems which are not detectable by routine monitoring.
-
Therefore, it will be crucial to have additional information to get a complete overview, especially in critically ill neonates in need of medical and respiratory support.
-
In this review, we offer additional information which can be provided by cerebral NIRS during fetal to neonatal transition.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
23 June 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41390-022-02173-x
References
Rudolph, A. M. Fetal and neonatal pulmonary circulation. Annu. Rev. Physiol. 41, 383–395 (1979).
Apgar, V. A proposal for a new method of evaluation of the newborn infant. Curr. Res Anesth. Analg. 32, 260–267 (1953).
Casey, B. M., McIntire, D. D. & Leveno, K. J. The continuing value of the Apgar score for the assessment of newborn infants. N. Engl. J. Med 344, 467–471 (2001).
O’Donnell, C. P., Kamlin, C. O., Davis, P. G., Carlin, J. B. & Morley, C. J. Clinical assessment of infant colour at delivery. Arch. Dis. Child Fetal Neonatal Ed. 92, F465–F467 (2007).
O’Donnell, C. P., Kamlin, C. O., Davis, P. G., Carlin, J. B. & Morley, C. J. Interobserver variability of the 5-minute Apgar score. J. Pediatr. 149, 486–489 (2006).
Madar, J. et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 161, 291–326 (2021).
Pichler, G., Cheung, P. Y., Aziz, K., Urlesberger, B. & Schmölzer, G. M. How to monitor the brain during immediate neonatal transition and resuscitation? A systematic qualitative review of the literature. Neonatology 105, 205–210 (2014).
van Bel, F., Lemmers, P. & Naulaers, G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology 94, 237–244 (2008).
Jobsis, F. F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198, 1264–1267 (1977).
Edwards, A. D. et al. Cotside measurement of cerebral blood flow in ill newborn infants by near infrared spectroscopy. Lancet 2, 770–771 (1988).
McCormick, P. W. et al. Noninvasive cerebral optical spectroscopy for monitoring cerebral oxygen delivery and hemodynamics. Crit. Care Med 19, 89–97 (1991).
Peebles, D. M. et al. Changes in human fetal cerebral oxygenation and blood volume during delivery. Am. J. Obstet. Gynecol. 167, 1916–1917 (1992).
Isobe, K. et al. Changes in cerebral hemoglobin concentration and oxygen saturation immediately after birth in the human neonate using full-spectrum near infrared spectroscopy. J. Biomed. Opt. 5, 283–286 (2000).
Isobe, K. et al. Measurement of cerebral oxygenation in neonates after vaginal delivery and cesarean section using full-spectrum near infrared spectroscopy. Comp. Biochem Physiol. A Mol. Integr. Physiol. 132, 133–138 (2002).
Fauchère, J. C. et al. Near-infrared spectroscopy measurements of cerebral oxygenation in newborns during immediate postnatal adaptation. J. Pediatr. 156, 372–376 (2010).
Urlesberger, B. et al. Regional oxygen saturation of the brain and peripheral tissue during birth transition of term infants. J. Pediatr. 157, 740–744 (2010).
Binder, C. et al. Cerebral and peripheral regional oxygen saturation during postnatal transition in preterm neonates. J. Pediatr. 163, 394–399 (2013).
Schwaberger, B. et al. Even mild respiratory distress alters tissue oxygenation significantly in preterm infants during neonatal transition. Physiol. Meas. 35, 2085–2099 (2014).
Pichler, G. et al. Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J. Pediatr. 163, 1558–1563 (2013).
Baik, N. et al. Reference Ranges for Cerebral Tissue Oxygen Saturation Index in Term Neonates during Immediate Neonatal Transition after Birth. Neonatology 108, 283–286 (2015).
Welsford, M. et al. International Liaison Committee on Resuscitation Neonatal Life Support Task Force: Initial Oxygen Use for Preterm Newborn Resuscitation: A Systematic Review With Meta-analysis. Pediatrics 143, e20181828 (2019).
Dawson, J. A. et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 125, e1340–e1347 (2010).
Baik, N. et al. Cerebral haemorrhage in preterm neonates: does cerebral regional oxygen saturation during the immediate transition matter? Arch. Dis. Child Fetal Neonatal Ed. 100, F422–F427 (2015).
Fuchs, H. et al. Brain oxygenation monitoring during neonatal resuscitation of very low birth weight infants. J. Perinatol. 32, 356–362 (2012).
Oei, J. L. et al. Outcomes of oxygen saturation targeting during delivery room stabilisation of preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 103, F446–F454 (2018).
Binder-Heschl, C. et al. Oxygen Saturation Targeting During Delivery Room Stabilization: What Does This Mean for Regional Cerebral Oxygenation? Front Pediatr. 7, 274 (2019).
Pichler, G. et al. Cerebral Oxygen Saturation to Guide Oxygen Delivery in Preterm Neonates for the Immediate Transition after Birth: A 2-Center Randomized Controlled Pilot Feasibility Trial. J. Pediatr. 170, 73–8.e1 (2016).
Kapadia, V. et al. Outcomes of delivery room resuscitation of bradycardic preterm infants: A retrospective cohort study of randomised trials of high vs low initial oxygen concentration and an individual patient data analysis. Resuscitation 167, 209–217 (2021).
Bresesti I. et al. Impact of bradycardia and hypoxemia on oxygenation in preterm infants requiring respiratory support at birth. Resuscitation 164, 62–69 (2021).
Schwaberger, B. et al. Transitional changes in cerebral blood volume at birth. Neonatology 108, 253–258 (2015).
Wyatt, J. S. et al. Response of cerebral blood volume to changes in arterial carbon dioxide tension in preterm and term infants. Pediatr. Res 29, 553–557 (1991).
Schwaberger, B. et al. Cerebral blood volume during neonatal transition in term and preterm infants with and without respiratory support. Front Pediatr. 6, 132 (2018).
Noori, S. et al. Transitional changes in cardiac and cerebral hemodynamics in term neonates at birth. J. Pediatr. 160, 943–948 (2012).
Fuchs, H. et al. Cerebral oxygenation in very low birth weight infants supported with sustained lung inflations after birth. Pediatr. Res 70, 176–180 (2011).
Schwaberger, B. et al. Do sustained lung inflations during neonatal resuscitation affect cerebral blood volume in preterm infants? a randomized controlled pilot study. PLoS One 10, e0138964 (2015).
Li, E. S., Cheung, P. Y., Pichler, G., Aziz, K. & Schmölzer, G. M. Respiratory function and near infrared spectroscopy recording during cardiopulmonary resuscitation in an extremely preterm newborn. Neonatology 105, 200–204 (2014).
Badurdeen, S. et al. Excess cerebral oxygen delivery follows return of spontaneous circulation in near-term asphyxiated lambs. Sci. Rep. 10, 16443–020 (2020).
Urlesberger, B. et al. A left-to-right shunt via the ductus arteriosus is associated with increased regional cerebral oxygen saturation during neonatal transition. Neonatology 103, 259–263 (2013).
Baik, N. et al. Foramen ovale (FO) - The underestimated sibling of ductus arteriosus (DA): Relevance during neonatal transition. Early Hum. Dev. 103, 137–140 (2016).
Baik, N. et al. Blood pressure during the immediate neonatal transition: is the mean arterial blood pressure relevant for the cerebral regional oxygenation? Neonatology 112, 97–102 (2017).
Naulaers, G. et al. Use of tissue oxygenation index and fractional tissue oxygen extraction as non-invasive parameters for cerebral oxygenation. A validation study in piglets. Neonatology 92, 120–126 (2007).
Wong, F. Y. et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121, e604–e611 (2008).
Seri, I., Rudas, G., Bors, Z., Kanyicska, B. & Tulassay, T. Effects of low-dose dopamine infusion on cardiovascular and renal functions, cerebral blood flow, and plasma catecholamine levels in sick preterm neonates. Pediatr. Res 34, 742–749 (1993).
Baik-Schneditz N. et al. Cardiac output and cerebral oxygenation in term neonates during neonatal transition. Children 8, 439 (2021).
Bruckner, M. et al. Cerebral and peripheral tissue oxygenation in stable neonates: Absent influence of cardiac function. Acta Paediatrica 109, 1560–1569 (2020).
Tamussino, A. et al. Low cerebral activity and cerebral oxygenation during immediate transition in term neonates-A prospective observational study. Resuscitation 103, 49–53 (2016).
Matterberger, C. et al. Blood glucose and cerebral tissue oxygenation immediately after birth-an observational study. J. Pediatr. 200, 19–23 (2018).
Bruckner M. et al. Association between regional tissue oxygenation and body temperature in term and preterm infants born by caesarean section. Children 7, 205 (2020).
Garite, T. J., Clark, R. & Thorp, J. A. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am. J. Obstet. Gynecol. 191, 481–487 (2004).
Baik-Schneditz, N. et al. Effect of intrauterine growth restriction on cerebral regional oxygen saturation in preterm and term neonates during immediate postnatal transition. Neonatology 117, 324–330 (2020).
Baschat, A. A. & Harman, C. R. Antenatal assessment of the growth restricted fetus. Curr. Opin. Obstet. Gynecol. 13, 161–168 (2001).
Katheria, A. C. et al. Measuring cardiac changes using electrical impedance during delayed cord clamping: a feasibility trial. Matern Health Neonatol. Perinatol. 1, 15–015 (2015).
Padilla-Sánchez, C. et al. Delayed vs immediate cord clamping changes oxygen saturation and heart rate patterns in the first minutes after birth. J. Pediatr. 227, 149–156.e1 (2020).
Katheria, A. C. et al. Delayed cord clamping in newborns born at term at risk for resuscitation: a feasibility randomized clinical trial. J. Pediatr. 187, 313–317.e1 (2017).
Baenziger, O. et al. The influence of the timing of cord clamping on postnatal cerebral oxygenation in preterm neonates: a randomized, controlled trial. Pediatrics 119, 455–459 (2007).
Finn D. et al. Clamping the Umbilical Cord in Premature Deliveries (CUPiD): Neuromonitoring in the Immediate Newborn Period in a Randomized, Controlled Trial of Preterm Infants Born at <32 Weeks of Gestation. J Pediatr 208, 121–126 (2019).
Wolfsberger, C. H. et al. Fetal Inflammatory Response Syndrome and Cerebral Oxygenation During Immediate Postnatal Transition in Preterm Neonates. Front Pediatr. 8, 401 (2020).
Pichler G. et al. Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): an investigator-initiated, randomized, multi-center, multi-national, clinical trial on additional cerebral tissue oxygen saturation monitoring combined with defined treatment guidelines versus standard monitoring and treatment as usual in premature infants during immediate transition: study protocol for a randomized controlled trial. Trials. 20, 178 (2019).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: NBS, BS, GP, BU. (II) Administrative support: IB, HF, IL, NB, GL, MV, CBH. (III) Data analysis and interpretation: All authors. (IV) Manuscript writing: All authors (V) Final approval of manuscript: All authors
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: all author names were interchanged and they have now been corrected.
Supplementary information
Rights and permissions
About this article
Cite this article
Baik-Schneditz, N., Schwaberger, B., Bresesti, I. et al. Fetal to neonatal transition: what additional information can be provided by cerebral near infrared spectroscopy?. Pediatr Res 96, 579–585 (2024). https://doi.org/10.1038/s41390-022-02081-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02081-0
This article is cited by
-
Brain oxygenation monitoring during neonatal stabilization and resuscitation and its potential for improving preterm infant outcomes: a systematic review and meta-analysis with Bayesian analysis
European Journal of Pediatrics (2025)
-
The effects of cerebral oximetry in mechanically ventilated newborns: a protocol for the SafeBoosC-IIIv randomised clinical trial
Trials (2023)