Abstract
Background
Immune signatures at birth could be associated with clinical outcomes and will improve our understanding of immunity prenatal programming.
Methods
Data come from 235 newborns from the cohort study NELA. Production of cytokines was determined using Luminex technology. Associations between cytokine concentrations with sex and season of birth were examined by multivariate regression models.
Results
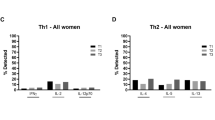
Umbilical cord blood cells produced high levels of inflammatory cytokines, moderate levels of Th1/Th2/Tr-related cytokines, and low levels of Th17 cytokines. Compared to females, male newborn cells secreted higher levels of Th2 (peptidoglycan-stimulated IL-13, odds ratio [OR] = 2.26; 95% CI 1.18, 4.31, p value = 0.013) and Th17 (polyinosinic:polycytidylic acid-stimulated IL-23, OR = 1.82, 95% CI 1.01, 3.27, p value = 0.046) and lower levels of Th1 (olive-stimulated IL-2, OR = 0.56, 95% CI 0.31, 0.99, p value = 0.047) cytokines. Also, children born during warm seasons showed decreased innate cytokine response to peptidoglycan (IL-6, OR = 0.28, 95% CI 0.15, 0.52, p value < 0.001) compared to those born in cold seasons; meanwhile, adaptive immunity cytokines were more frequently secreted by children born during warm seasons in response to allergen extracts (IL-10, OR = 2.11, 95% CI 1.12, 3.96, p value = 0.020; IL-17F, OR = 3.31, 95% CI 1.83, 5.99, p value < 0.001).
Conclusion
Newborns showed specific cytokines signatures influenced by sex and season of birth.
Impact
-
There is a limited number of population-based studies on the immune status at birth and the influence of prenatal and perinatal factors on it.
-
Characterization of cytokine signatures at birth related to the prenatal environment could improve our understanding of immunity prenatal programming.
-
Newborns exhibit specific unstimulated and stimulated cytokine signatures influenced by sex and season of birth.
-
Unstimulated and stimulated cytokine signatures in newborns may be associated with the development of related clinical outcomes later in life.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
28 November 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41390-022-02395-z
References
Schirmer, M., Kumar, V., Netea, M. G. & Xavier, R. J. The causes and consequences of variation in human cytokine production in health. Curr. Opin. Immunol. 54, 50–58 (2018).
Duijts, L. Fetal and infant origins of asthma. Eur. J. Epidemiol. 27, 5–14 (2012).
ter Horst, R. et al. Host and environmental factors influencing individual human cytokine responses. Cell 167, 1111–1124.e13 (2016).
Bakker, O. B. et al. Integration of multi-omics data and deep phenotyping enables prediction of cytokine responses. Nat. Immunol. 19, 776–786 (2018).
Decker, M.-L., Gotta, V., Wellmann, S. & Ritz, N. Cytokine profiling in healthy children shows association of age with cytokine concentrations. Sci. Rep. 7, 17842 (2017).
Bergroth, E. et al. Enhanced T helper 1 and 2 cytokine responses at birth associate with lower risk of middle ear infections in infancy. Pediatr. Allergy Immunol. 28, 53–59 (2017).
Chu, X. et al. Integration of metabolomics, genomics, and immune phenotypes reveals the causal roles of metabolites in disease. Genome Biol. 22, 198 (2021).
Hammad, H. & Lambrecht, B. N. The basic immunology of asthma. Cell 184, 2521–2522 (2021).
Gold, D. R. et al. Parental characteristics, somatic fetal growth, and season of birth influence innate and adaptive cord blood cytokine responses. J. Allergy Clin. Immunol. 124, 1078–1087 (2009).
Schaub, B. et al. Impairment of T helper and T regulatory cell responses at birth. Allergy 63, 1438–1447 (2008).
Thysen, A. H. et al. Season of birth shapes neonatal immune function. J. Allergy Clin. Immunol. 137, 1238–1246.e13 (2016).
Wood, R. A. et al. Relationships among environmental exposures, cord blood cytokine responses, allergy, and wheeze at 1 year of age in an inner-city birth cohort (Urban Environment and Childhood Asthma study). J. Allergy Clin. Immunol. 127, 913–919.e6 (2011).
Williams, T. J., Jones, C. A., Miles, E. A., Warner, J. O. & Warner, J. A. Fetal and neonatal IL-13 production during pregnancy and at birth and subsequent development of atopic symptoms. J. Allergy Clin. Immunol. 105, 951–959 (2000).
Macaubas, C. et al. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet 362, 1192–1197 (2003).
Prescott, S. L. et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J. Immunol. 160, 4730–4737 (1998).
Adel-Patient, K. et al. A comprehensive analysis of immune constituents in blood and bronchoalveolar lavage allows identification of an immune signature of severe asthma in children. Front. Immunol. 12, 700521 (2021).
Fitzpatrick, A. M., Higgins, M., Holguin, F., Brown, L. A. S. & Teague, W. G. The molecular phenotype of severe asthma in children. J. Allergy Clin. Immunol. 125, 851–857.e18 (2010).
Li, Y. et al. A functional genomics approach to understand variation in cytokine production in humans. Cell 167, 1099–1110.e14 (2016).
Dingle, K., Zimek, A., Azizieh, F. & Ansari, A. R. Establishing a many-cytokine signature via multivariate anomaly detection. Sci. Rep. 9, 9684 (2019).
García-Serna, A. M., Martín-Orozco, E., Hernández-Caselles, T. & Morales, E. Prenatal and perinatal environmental influences shaping the neonatal immune system: a focus on asthma and allergy origins. Int. J. Environ. Res. Public Health 18, 3962 (2021).
Chowdhury, N. U., Guntur, V. P., Newcomb, D. C. & Wechsler, M. E. Sex and gender in asthma. Eur. Respiratory Rev. 30, 210067 (2021).
Almqvist, C. et al. Season of birth, childhood asthma and allergy in a nationwide cohort—mediation through lower respiratory infections. Clin. Exp. Allergy 50, 222–230 (2020).
Morales, E. et al. The Nutrition in Early Life and Asthma (NELA) birth cohort study: rationale, design, and methods. Paediatr. Perinat. Epidemiol. 36, 310–324. https://doi.org/10.1111/ppe.12826 (2022).
López, M. C., Palmer, B. E. & Lawrence, D. A. Naïve T cells, unconventional NK and NKT cells, and highly responsive monocyte-derived macrophages characterize human cord blood. Immunobiology 219, 756–765 (2014).
García‐Serna, A. M. et al. Cytokine profiles in cord blood in relation to prenatal traffic‐related air pollution: The NELA cohort. Pediatr. Allergy Immunol. 33, e13732 (2022).
Wurfel, M. M. et al. Identification of high and low responders to lipopolysaccharide in normal subjects: an unbiased approach to identify modulators of innate immunity. J. Immunol. 175, 2570–2578 (2005).
Zhang, X., Zhivaki, D. & Lo-Man, R. Unique aspects of the perinatal immune system. Nat. Rev. Immunol. 17, 495–507 (2017).
Razzaghian, H. R. et al. Neonatal T helper 17 responses are skewed towards an immunoregulatory interleukin-22 phenotype. Front. Immunol. 12, 655027 (2021).
McGeachy, M. J., Cua, D. J. & Gaffen, S. L. The IL-17 family of cytokines in health and disease. Immunity 50, 892–906 (2019).
Ridolo, E. et al. Sex in respiratory and skin allergies. Clin. Rev. Allergy Immunol. 56, 322–332 (2019).
Jackson, D. J. et al. Serum IL-6: a biomarker in childhood asthma? J. Allergy Clin. Immunol. 145, 1701–1704.e3 (2020).
Prescott, S. L. et al. Presymptomatic differences in Toll-like receptor function in infants who have allergy. J. Allergy Clin. Immunol. 122, 391–399.e5 (2008).
Mantovani, A., Dinarello, C. A., Molgora, M. & Garlanda, C. Interleukin−1 and related cytokines in the regulation of inflammation and immunity. Immunity 50, 778–795 (2019).
Disanto, G. et al. Month of birth, vitamin D and risk of immune-mediated disease: a case control study. BMC Med. 10, 69 (2012).
Dobson, R., Giovannoni, G. & Ramagopalan, S. The month of birth effect in multiple sclerosis: systematic review, meta-analysis and effect of latitude. J. Neurol. Neurosurg. Psychiatry 84, 427–432 (2013).
Kahn, H. S. et al. Association of type 1 diabetes with month of birth among U.S. youth. Diabetes Care 32, 2010–2015 (2009).
Knudsen, T. B. et al. Season of birth and risk of atopic disease among children and adolescents. J. Asthma 44, 257–260 (2007).
Nilsson, L. et al. Season of birth as predictor of atopic manifestations. Arch. Dis. Child. 76, 341–344 (1997).
Matsui, T. et al. Food allergy is linked to season of birth, sun exposure, and vitamin D deficiency. Allergol. Int. 68, 172–177 (2019).
Watad, A. et al. Seasonality and autoimmune diseases: the contribution of the four seasons to the mosaic of autoimmunity. J. Autoimmun. 82, 13–30 (2017).
Dopico, X. C. et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat. Commun. 6, 7000 (2015).
Sullivan Dillie, K. T. et al. The influence of processing factors and non-atopy-related maternal and neonate characteristics on yield and cytokine responses of cord blood mononuclear cells. Clin. Exp. Allergy 38, 298–304 (2007).
Acknowledgements
We are extremely grateful to all the families who participate in this study, the hospital technicians, midwives, and gynecologists for recruitment and following them up, and the whole NELA team for their commitment and their role in the success of the study. We want to particularly acknowledge the BioBank “Biobanco en Red de la Región de Murcia” (PT17/0015/0038 and PT20/00109) integrated with the Spanish National Biobanks Network (B.000859) for its collaboration. We acknowledge Dr José Joaquín Cerón and Dr Luis Pardo Marín for their support with the cytokine determination and Dr Manuel Sanchez Angulo for the critical reading of the manuscript and helpful suggestions. Finally, we thank Francisco Arques for his excellent technical support.
Funding
This study has been funded by the Instituto de Salud Carlos III (ISCIII) through the projects CP14/00046, PIE15/00051, PI16/00422, PI19/00863 and ARADyAL network RD160006, and co-funded by the European Union. A.M.G.-S. was funded by a predoctoral Fellowship (FI17/00086) and E.M. was funded by Miguel Servet Fellowships (MS14/00046 and CPII19/00019) awarded by the Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Science, Innovation and Universities, and Fondos FEDER. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
Conception and design of the study: A.M.G.-S., E.M., L.G.-M., and E.M.-O. Acquisition and analysis of data: A.M.G.-S., E.M., E.C.-C., M.N.-M., M.A.G.-B., J.V.-M., T.H.-C., V.P.-F., A.E.M.-T., and E.M.-O. Drafting of the manuscript: E.M. and E.M.-O. Critical revision and final approval of the version to be published: A.M.G.-S., E.M., E.C.-C., M.N.-M., M.A.G.-B., J.V.-M., T.H.-C., V.P.-F., A.E.M.-T., L.G.-M., and E.M.-O.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Parental consent of subjects was required prior to enrollment in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the funding information has been corrected.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garcia-Serna, A.M., Morales, E., Cantero-Cano, E. et al. Cytokine production by newborns: influence of sex and season of birth. Pediatr Res 93, 526–534 (2023). https://doi.org/10.1038/s41390-022-02153-1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02153-1