Abstract
Background
Examine the real-world clinical impact of adopting less invasive surfactant administration (LISA) as the primary surfactant administration method in extremely preterm infants.
Methods
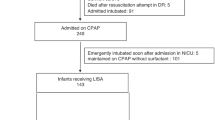
Single-center pre-post cohort study conducted over a 4-year period comparing outcomes of spontaneously breathing inborn infants 24+0–28+6 weeks gestational age (GA) receiving surfactant via endotracheal tube (pre-cohort, n = 154) or LISA via thin catheter (post-cohort, n = 70). Primary outcome was need for invasive mechanical ventilation (IMV, ≥2 h) ≤72 h of age. Secondary outcomes were a composite of mortality, bronchopulmonary dysplasia, intraventricular hemorrhage ≥grade 3 or necrotizing enterocolitis, and its individual components. Groups were compared using propensity score methods, including covariates: GA, birth weight, sex, small for GA, SNAP II ≥20, premature rupture of membranes, maternal hypertension/diabetes, and C-section.
Results
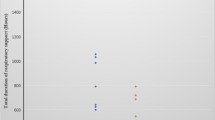
GA and birth weight were 27.1 (26, 28.1) weeks and 914 (230) g, and 27.1 (26.1, 28.1) weeks and 920 (236) g for pre- and post-cohorts, respectively. Pre-cohort had higher C-section rates, (67% vs. 51%, p = 0.03). After adjustment for covariates, LISA was associated with reduced IMV exposure [AOR (95% CI) 0.07 (0.04, 0.11)], lower odds of the composite clinical outcome [0.49 (0.33, 0.73)], and most of its individual components.
Conclusion
Real-world experience favors LISA as the primary method in extremely preterm infants with established spontaneous respiration.
Impact
-
Less invasive surfactant administration (LISA) is associated with a reduction in respiratory morbidity, but real-world data of routine use among extremely preterm infants are limited.
-
LISA is associated with reduced frequency of exposure to and duration of IMV in both ≤72 h after birth and during hospital stay.
-
LISA is associated with a reduction in mortality, and most other major morbidities including bronchopulmonary dysplasia, and interventricular hemorrhage.
-
Data from a large North American center providing real-world clinical outcomes following LISA as the primary method of surfactant administration.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The dataset generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rojas-Reyes, M. X., Morley, C. J. & Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. CD000510 (2012).
Owen, L. S., Manley, B. J., Davis, P. G. & Doyle, L. W. The evolution of modern respiratory care for preterm infants. Lancet 389, 1649–1659 (2017).
Kalikkot Thekkeveedu, R., Guaman, M. C. & Shivanna, B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir. Med. 132, 170–177 (2017).
Stevens, T. P., Harrington, E. W., Blennow, M. & Soll, R. F. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst. Rev. CD003063 (2007).
Herting, E., Hartel, C. & Gopel, W. Less invasive surfactant administration (LISA): chances and limitations. Arch. Dis. Child. Fetal Neonatal Ed. 104, F655–F659 (2019).
Dargaville, P. A. et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch. Dis. Child. Fetal Neonatal Ed. 98, F122–F126 (2013).
Kribs, A. et al. Surfactant without intubation in preterm infants with respiratory distress: first multi-center data. Klinische Padiatrie. 222, 13–17 (2010).
Gopel, W. et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 378, 1627–1634 (2011).
Kanmaz, H. G., Erdeve, O., Canpolat, F. E., Mutlu, B. & Dilmen, U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics 131, e502–e509 (2013).
Klebermass-Schrehof, K. et al. Less invasive surfactant administration in extremely preterm infants: impact on mortality and morbidity. Neonatology 103, 252–258 (2013).
Kribs, A. et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 169, 723–730 (2015).
Hartel, C. et al. Less invasive surfactant administration and complications of preterm birth. Sci. Rep. 8, 8333 (2018).
Aldana-Aguirre, J. C., Pinto, M., Featherstone, R. M. & Kumar, M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 102, F17–F23 (2017).
Gopel, W. et al. Less invasive surfactant administration is associated with improved pulmonary outcomes in spontaneously breathing preterm infants. Acta Paediatr. 104, 241–246 (2015).
Isayama, T., Iwami, H., McDonald, S. & Beyene, J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA 316, 611–624 (2016).
Williamson, S. L., McDermott, H. & Gowda H. Implementing less invasive surfactant administration on a neonatal unit. Arch. Dis. Child Educ. Pract. Ed. edpract-2020-320574 (2021).
Roberts, C. T. et al. Outcomes after introduction of minimally invasive surfactant therapy in two Australian tertiary neonatal units. J. Pediatr. 229, 141–146 (2021).
Conlon, S. M. et al. Introducing less-invasive surfactant administration into a level IV NICU: a quality improvement initiative. Children 8, 580 (2021).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. care Med. 163, 1723–1729 (2001).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
Walsh, M. C. & Kliegman, R. M. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr. Clin. North Am. 33, 179–201 (1986).
Bellos, I., Fitrou, G., Panza, R. & Pandita, A. Comparative efficacy of methods for surfactant administration: a network meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 106, 474–487 (2021).
Sweet, D. G. et al. European consensus guidelines on the management of respiratory distress syndrome – 2019 update. Neonatology 115, 432–450 (2019).
Szczapa, T., Hozejowski, R., Krajewski, P. & Study, G. Implementation of less invasive surfactant administration in clinical practice-Experience of a mid-sized country. PLoS One 15, e0235363 (2020).
Berneau, P., Nguyen Phuc Thu, T., Pladys, P. & Beuchee, A. Impact of surfactant administration through a thin catheter in the delivery room: a quality control chart analysis coupled with a propensity score matched cohort study in preterm infants. PLoS One 13, e0208252 (2018).
Elbaz, Y., Portnov, I., Lurie-Marcu, B. & Shinwell, E. S. Minimally invasive surfactant therapy versus intubation for surfactant delivery in preterm infant with RDS: evaluation of safety and efficacy. J. Matern. Fetal Neonatal Med. 1–5 (2021).
Shetty, S. et al. Less invasive surfactant administration in very prematurely born infants. AJP Rep. 11, e119–e122 (2021).
Perez-Iranzo, A., Jarque, A., Toledo, J. D. & Tosca, R. Less invasive surfactant administration reduces incidence of severe intraventricular haemorrage in preterms with respiratory distress syndrome: a cohort study. J. Perinatol. 40, 1185–1192 (2020).
Bugter, I. A. L. et al. Introduction of less invasive surfactant administration (LISA), impact on diagnostic and therapeutic procedures in early life: a historical cohort study. BMC Pediatr. 20, 421 (2020).
Ramos-Navarro, C., Sanchez-Luna, M., Zeballos-Sarrato, S. & Gonzalez-Pacheco, N. Three-year perinatal outcomes of less invasive beractant administration in preterm infants with respiratory distress syndrome. J. Matern. Fetal Neonatal Med. 33, 2704–2710 (2020).
Dargaville, P. A., Ali, S. K. M., Jackson, H. D., Williams, C. & De Paoli, A. G. Impact of minimally invasive surfactant therapy in preterm infants at 29-32 weeks gestation. Neonatology 113, 7–14 (2018).
Author information
Authors and Affiliations
Contributions
A.J. conceived the research question, conceptualized the initial study design and data collection forms, and revised the final draft of the manuscript. M.B. participated in the initial study design and data collection forms, collected in-hospital data for the study cohort, and produced the first draft of the manuscript. V.D. reviewed the study protocol, collected in-hospital data for the study cohort, reviewed the manuscript, and provided critical feedback. L.H. reviewed the study protocol, collected in-hospital data for the study cohort, reviewed the manuscript, and provided critical feedback. B.L. reviewed the study protocol, collected in-hospital data for the study cohort, reviewed the manuscript, and provided critical feedback. X.Y.Y. performed all statistical analysis for the study, reviewed the manuscript, and provided critical feedback. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baczynski, M., Deekonda, V., Hamilton, L. et al. Clinical impact of less invasive surfactant administration using video laryngoscopy in extremely preterm infants. Pediatr Res 93, 990–995 (2023). https://doi.org/10.1038/s41390-022-02197-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02197-3