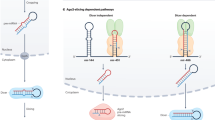
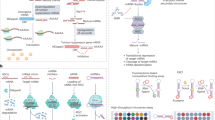
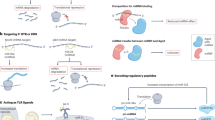
Abstract
In the past decade, growing interest in micro-ribonucleic acids (miRNAs) has catapulted these small, non-coding nucleic acids to the forefront of biomarker research. Advances in scientific knowledge have made it clear that miRNAs play a vital role in regulating cellular physiology throughout the human body. Perturbations in miRNA signaling have also been described in a variety of pediatric conditions—from cancer, to renal failure, to traumatic brain injury. Likewise, the number of studies across pediatric disciplines that pair patient miRNA-omics with longitudinal clinical data are growing. Analyses of these voluminous, multivariate data sets require understanding of pediatric phenotypic data, data science, and genomics. Use of machine learning techniques to aid in biomarker detection have helped decipher background noise from biologically meaningful changes in the data. Further, emerging research suggests that miRNAs may have potential as therapeutic targets for pediatric precision care. Here, we review current miRNA biomarkers of pediatric diseases and studies that have combined machine learning techniques, miRNA-omics, and patient health data to identify novel biomarkers and potential therapeutics for pediatric diseases.
Impact
-
In the following review article, we summarized how recent developments in microRNA research may be coupled with machine learning techniques to advance pediatric precision care.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Almeida, M. I., Reis, R. M. & Calin, G. A. MicroRNA history: discovery, recent applications, and next frontiers. Mutat. Res. Mol. Mech. Mutagen. 717, 1–8 (2011).
Weber, J. A. et al. The microRNA spectrum in 12 body fluids. Clin. Chem. 56, 1733–1741 (2010).
Fehlmann, T., Ludwig, N., Backes, C., Meese, E. & Keller, A. Distribution of microRNA biomarker candidates in solid tissues and body fluids. RNA Biol. 13, 1084 (2016).
Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 12, 861–874 (2011).
Mohr, A. M. & Mott, J. L. Overview of microRNA biology. Semin. Liver Dis. 35, 3 (2015).
Newman, M. A. & Hammond, S. M. Emerging paradigms of regulated microRNA processing. Genes Dev. 24, 1086–1092 (2010).
Creugny, A., Fender, A. & Pfeffer, S. Regulation of primary microRNA processing. FEBS Lett. 592, 1980–1996 (2018).
Fiorenza, A. & Barco, A. Role of Dicer and the miRNA system in neuronal plasticity and brain function. Neurobiol. Learn. Mem. 135, 3–12 (2016).
Kobayashi, H. & Tomari, Y. RISC assembly: coordination between small RNAs and Argonaute proteins. Biochim. Biophys. Acta Gene Regul. Mech. 1859, 71–81 (2016).
Paul, S. et al. Roles of microRNAs in chronic pediatric diseases and their use as potential biomarkers: a review. Arch. Biochem. Biophys. 699, 108763 (2021).
Simonson, B. & Das, S. MicroRNA therapeutics: the next magic bullet? Mini Rev. Med. Chem. 15, 467 (2015).
NCBI. Newborn screening - understanding genetics - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK132148/ (2022).
Wood, S. K. & Sperling, R. Pediatric screening: development, anemia, and lead. Prim. Care Clin. Pract. 46, 69–84 (2019).
Kanji, A., Khoza-Shangase, K. & Moroe, N. Newborn hearing screening protocols and their outcomes: a systematic review. Int. J. Pediatr. Otorhinolaryngol. 115, 104–109 (2018).
Tishkowski, K. & Gupta, V. in Laboratory Hematology Practice (eds Kottke-Marchant, K. & Davis, B. H.) 638–646 (Wiley, 2021).
American Family Physician. Clinical utility of the erythrocyte sedimentation rate. https://www.aafp.org/afp/1999/1001/p1443.html (2022).
Bock, C. Epigenetic biomarker development. Epigenomics 1, 99–110 (2009).
Phillips, K. A., Van Bebber, S. & Issa, A. M. Diagnostics and biomarker development: priming the pipeline. Nat. Rev. Drug Discov. 5, 463–469 (2006).
Kuzhandai Velu, V., Ramesh, R. & Srinivasan, A. R. Circulating microRNAs as biomarkers in health and disease. J. Clin. Diagn. Res. 6, 1791 (2012).
Jung, M. et al. Robust microRNA stability in degraded RNA preparations from human tissue and cell samples. Clin. Chem. 56, 998–1006 (2010).
Matsumoto, J., Stewart, T., Banks, W. A. & Zhang, J. The transport mechanism of extracellular vesicles at the blood-brain barrier. Curr. Pharm. Des. 23, 6206–6214 (2017).
Zhang, J. et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics 13, 17 (2015).
Cha, D. J. et al. miR-212 and miR-132 are downregulated in neurally derived plasma exosomes of Alzheimer’s patients. Front. Neurosci. 13, 1208 (2019).
Andrews, W. J., Brown, E. D., Dellett, M., Hogg, R. E. & Simpson, D. A. Rapid quantification of microRNAs in plasma using a fast real-time PCR system. Biotechniques 58, 244–252 (2015).
Scott, G. K., Mattie, M. D., Berger, C. E., Benz, S. C. & Benz, C. C. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res. 66, 1277–1281 (2006).
LaRocca, D. et al. Comparison of serum and saliva miRNAs for identification and characterization of mTBI in adult mixed martial arts fighters. PLoS ONE 14, e0207785 (2019).
Zwaigenbaum, L. & Maguire, J. Autism screening: where do we go from here? Pediatrics 144, e20190925 (2019).
Hicks, S. D. & Middleton, F. A. A comparative review of microRNA expression patterns in autism spectrum disorder. Front. Psychiatry 7, 176 (2016).
Ozkul, Y. et al. A heritable profile of six miRNAs in autistic patients and mouse models. Sci. Rep. 10, 9011–9011 (2020).
Hicks, S. D., Ignacio, C., Gentile, K. & Middleton, F. A. Salivary miRNA profiles identify children with autism spectrum disorder, correlate with adaptive behavior, and implicate ASD candidate genes involved in neurodevelopment. BMC Pediatr. 16, 1–11 (2016).
Mor, M., Nardone, S., Sams, D. S. & Elliott, E. Hypomethylation of miR-142 promoter and upregulation of microRNAs that target the oxytocin receptor gene in the autism prefrontal cortex. Mol. Autism 6, 1–11 (2015).
Hicks, S. D. et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J. Am. Acad. Child Adolesc. Psychiatry 59, 296–308 (2020).
Hicks, S. D. et al. Validation of a salivary RNA test for childhood autism spectrum disorder. Front. Genet. 9, 534 (2018).
Vogt, B., Falkenberg, C., Weiler, N. & Frerichs, I. Pulmonary function testing in children and infants. Physiol. Meas. 35, R59 (2014).
Simpson, L. J. et al. A miRNA upregulated in asthma airway T cells promotes TH2 cytokine production. Nat. Immunol. 15, 1162 (2014).
Mattes, J., Collison, A., Plank, M., Phipps, S. & Foster, P. S. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc. Natl Acad. Sci. USA 106, 18704 (2009).
Panganiban, R. P. L. et al. Differential microRNA epression in asthma and the role of miR-1248 in regulation of IL-5. Am. J. Clin. Exp. Immunol. 1, 154 (2012).
Liu, F., Qin, H. B., Xu, B., Zhou, H. & Zhao, D. Y. Profiling of miRNAs in pediatric asthma: upregulation of miRNA-221 and miRNA-485-3p. Mol. Med. Rep. 6, 1178–1182 (2012).
Kho, A. T. et al. Circulating microRNAs and prediction of asthma exacerbation in childhood asthma. Respir. Res. 19, 1–9 (2018).
Mendes, F. C. et al. Development and validation of exhaled breath condensate microRNAs to identify and endotype asthma in children. PLoS ONE 14, e0224983 (2019).
Lumba-Brown, A. et al. Centers for disease control and prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 172, e182853 (2018).
Atif, H. & Hicks, S. D. A review of microRNA biomarkers in traumatic brain injury. J. Exp. Neurosci. 13, 117906951983228 (2019).
Di Pietro, V. et al. Unique diagnostic signatures of concussion in the saliva of male athletes: the Study of Concussion in Rugby Union through MicroRNAs (SCRUM). Br. J. Sports Med. 55, 1395–1404 (2021).
Johnson, J. J. et al. Association of salivary microRNA changes with prolonged concussion symptoms. JAMA Pediatr. 172, 65–73 (2018).
Hicks, S. D. et al. Diagnosing mild traumatic brain injury using saliva RNA compared to cognitive and balance testing. Clin. Transl. Med. 10, e197 (2020).
Lee, D. K., Chang, V. Y., Kee, T., Ho, C. M. & Ho, D. Optimizing combination therapy for acute lymphoblastic leukemia using a phenotypic personalized medicine digital health platform: retrospective optimization individualizes patient regimens to maximize efficacy and safety. SLAS Technol. 22, 276–288 (2017).
Wallaert, A. et al. Comprehensive miRNA expression profiling in human T-cell acute lymphoblastic leukemia by small RNA-sequencing. Sci. Rep. 7, 1–8 (2017).
Lilljebjörn, H. & Fioretos, T. New oncogenic subtypes in pediatric B-cell precursor acute lymphoblastic leukemia. Blood 130, 1395–1401 (2017).
Luan, C., Yang, Z. & Chen, B. The functional role of microRNA in acute lymphoblastic leukemia: relevance for diagnosis, differential diagnosis, prognosis, and therapy. Onco Targets Ther. 8, 2903 (2015).
Egyed, B. et al. MicroRNA-181a as novel liquid biopsy marker of central nervous system involvement in pediatric acute lymphoblastic leukemia. J. Transl. Med. 18, 250 (2020).
Tong, N. et al. Hsa-miR-196a2 polymorphism increases the risk of acute lymphoblastic leukemia in Chinese children. Mutat. Res. Mol. Mech. Mutagen. 759, 16–21 (2014).
de Oliveira, J. C., Brassesco, M. S., Scrideli, C. A., Tone, L. G. & Narendran, A. MicroRNA expression and activity in pediatric acute lymphoblastic leukemia (ALL). Pediatr. Blood Cancer 59, 599–604 (2012).
Ultimo, S. et al. Roles and clinical implications of microRNAs in acute lymphoblastic leukemia. J. Cell. Physiol. 233, 5642–5654 (2018).
Sellin, J. H. & Shah, R. R. The promise and pitfalls of serologic testing in inflammatory bowel disease. Gastroenterol. Clin. North Am. 41, 463–482 (2012).
Ghafouri-Fard, S., Eghtedarian, R. & Taheri, M. The crucial role of non-coding RNAs in the pathophysiology of inflammatory bowel disease. Biomed. Pharmacother. 129, 110507 (2020).
Chen, P. et al. Circulating microRNA146b-5p is superior to C-reactive protein as a novel biomarker for monitoring inflammatory bowel disease. Aliment. Pharmacol. Ther. 49, 733–743 (2019).
Koukos, G. et al. MicroRNA-124 regulates STAT3 expression and is downregulated in colon tissues of pediatric patients with ulcerative colitis. Gastroenterology 145, 842 (2013).
Iborra, M. et al. Identification of serum and tissue micro-RNA expression profiles in different stages of inflammatory bowel disease. Clin. Exp. Immunol. 173, 250 (2013).
Mohammadi, A., Kelly, O. B., Smith, M. I., Kabakchiev, B. & Silverberg, M. S. Differential miRNA expression in ileal and colonic tissues reveals an altered immunoregulatory molecular profile in individuals with Crohn’s disease versus healthy subjects. J. Crohns Colitis 13, 1459 (2019).
Valmiki, S., Ahuja, V. & Paul, J. MicroRNA exhibit altered expression in the inflamed colonic mucosa of ulcerative colitis patients. World J. Gastroenterol. 23, 5324 (2017).
Patterson, C. C. et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 157, 107842 (2019).
Marchand, L. et al. MiRNA-375 a sensor of glucotoxicity is altered in the serum of children with newly diagnosed type 1 diabetes. J. Diabetes Res. 2016, 1869082 (2016).
Åkerman, L., Casas, R., Ludvigsson, J., Tavira, B. & Skoglund, C. Serum miRNA levels are related to glucose homeostasis and islet autoantibodies in children with high risk for type 1 diabetes. PLoS ONE 13, e0191067 (2018).
Bertoccini, L. et al. Circulating miRNA-375 levels are increased in autoantibodies-positive first-degree relatives of type 1 diabetes patients. Acta Diabetol. 56, 707–710 (2019).
Nielsen, L. B. et al. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp. Diabetes Res. 2012, 896362 (2012).
Abdelghaffar, S. et al. MicroRNAs and risk factors for diabetic nephropathy in Egyptian children and adolescents with type 1 diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 13, 2485–2494 (2020).
Zhong, X. Q. et al. Umbilical cord blood-derived exosomes from very preterm infants with bronchopulmonary dysplasia impaired endothelial angiogenesis: roles of exosomal microRNAs. Front. Cell Dev. Biol. 9, 529 (2021).
Nardiello, C. & Morty, R. E. MicroRNA in late lung development and bronchopulmonary dysplasia: the need to demonstrate causality. Mol. Cell. Pediatr. 3, 1–7 (2016).
Rogers, L. K. et al. Attenuation of MIR-17∼92 cluster in bronchopulmonary dysplasia. Ann. Am. Thorac. Soc. 12, 1506–1513 (2015).
Lal, C. V. et al. Exosomal microRNA predicts and protects against severe bronchopulmonary dysplasia in extremely premature infants. JCI Insight 3, e93994 (2018).
Oji-Mmuo, C. N. et al. Tracheal aspirate transcriptomic and miRNA signatures of extreme premature birth with bronchopulmonary dysplasia. J. Perinatol. 41, 551–561 (2020).
Siddaiah, R. et al. MicroRNA signatures associated with bronchopulmonary dysplasia severity in tracheal aspirates of preterm infants. Biomedicines 9, 1–16 (2021).
Chen, X., Xie, D., Zhao, Q. & You, Z. H. MicroRNAs and complex diseases: from experimental results to computational models. Brief. Bioinform. 20, 515–539 (2019).
Chen, L. et al. Trends in the development of miRNA bioinformatics tools. Brief. Bioinform. 20, 1836–1852 (2019).
Varma, M. et al. Outgroup machine learning approach identifies single nucleotide variants in noncoding DNA associated with autism spectrum disorder. Pac. Symp. Biocomput. 24, 260 (2019).
Min, N. et al. Circulating salivary miRNA hsa-miR-221 as clinically validated diagnostic marker for hand, foot, and mouth disease in pediatric patients. EBioMedicine 31, 299 (2018).
Chiaretti, S., Zini, G. & Bassan, R. Diagnosis and subclassification of acute lymphoblastic leukemia. Mediterr. J. Hematol. Infect. Dis. 6, 2014073 (2014).
Almeida, R. S. et al. MicroRNA expression profiles discriminate childhood T- from B-acute lymphoblastic leukemia. Hematol. Oncol. 37, 103–112 (2019).
Bozzola, M. et al. in Celiac Disease - From Bench to the Clinic (eds Rodrigo, L. & Hernández-Lahoz, C.) Ch. 4 (IntechOpen, 2018).
Comincini, S. et al. Identification of autophagy-related genes and their regulatory miRNAs associated with celiac disease in children. Int. J. Mol. Sci. 18, 391 (2017).
Casalino, G. et al. in Lecture Notes in Computer Science 177–188 (Springer Verlag, 2019).
Casalino, G., Castellano, G., Consiglio, A., Nuzziello, N. & Vessio, G. MicroRNA expression classification for pediatric multiple sclerosis identification. J. Ambient Intell. Humaniz. Comput. 1, 1–10 (2021).
Geurts, P., Ernst, D. & Wehenkel, L. Extremely randomized trees. Mach. Learn. 63, 3–42 (2006).
Zheng, K. et al. MLMDA: a machine learning approach to predict and validate MicroRNA-disease associations by integrating of heterogenous information sources. J. Transl. Med. 17, 1–14 (2019).
Li, X., Zhu, D. & Levy, P. Predicting clinical outcomes with patient stratification via deep mixture neural networks. AMIA Summits Transl. Sci. Proc. 2020, 367 (2020).
Shrikumar, A., Greenside, P. & Kundaje, A. Learning important features through propagating activation differences. PMLR 70, 3145–3153 (2017).
Sundararajan, M., Taly, A. & Yan, Q. Axiomatic attribution for deep networks. PMLR 70, 3319–3328 (2017).
Pan, D., Li, X. & Zhu, D. Explaining deep neural network models with adversarial gradient integration. In Thirtieth International Joint Conference on Artificial Intelligence 2876–2883 (IJCAI, 2021).
Van Der Ree, M. H. et al. Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antivir. Res. 111, 53–59 (2014).
van Zandwijk, N. et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 18, 1386–1396 (2017).
Omachi, K. & Miner, J. H. Alport syndrome therapeutics: ready for prime-time players. Trends Pharmacol. Sci. 40, 803–806 (2019).
Ottosen, S. et al. In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrob. Agents Chemother. 59, 599 (2015).
Kuntzen, T. et al. Naturally occurring dominant resistance mutations to hepatitis C virus protease and polymerase inhibitors in treatment-naïve patients. Hepatology 48, 1769–1778 (2008).
Pottoo, F. H., Javed, M. N., Rahman, J. U., Abu-Izneid, T. & Khan, F. A. Targeted delivery of miRNA based therapeuticals in the clinical management of glioblastoma multiforme. Semin. Cancer Biol. 69, 391–398 (2021).
Sukumar, U. K. et al. Intranasal delivery of targeted polyfunctional gold-iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials 218, 119342 (2019).
Bertucci, A. et al. Combined DELIVERY OF TEMOZOLOMIDE AND Anti-miR221 PNA using mesoporous silica nanoparticles induces apoptosis in resistant glioma cells. Small 11, 5687–5695 (2015).
Cao, C. et al. The long intergenic noncoding RNA UFC1, a target of microRNA 34a, interacts with the mRNA stabilizing protein HuR to increase levels of β-catenin in HCC cells. Gastroenterology 148, 415.e18–426.e18 (2015).
Gougelet, A. et al. Antitumour activity of an inhibitor of miR-34a in liver cancer with β-catenin-mutations. Gut 65, 1024–1034 (2016).
Hong, D. S. et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 122, 1630–1637 (2020).
Guo, J. et al. Dysregulated expression of microRNA-21 and disease-related genes in human patients and in a mouse model of Alport syndrome. Hum. Gene Ther. 30, 865–881 (2019).
Gomez, I. G. et al. Anti–microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J. Clin. Investig. 125, 141 (2015).
Hunt, A. M. D., Glasgow, A. M. A., Humphreys, H. & Greene, C. M. Alpha-1 antitrypsin—a target for microRNA-based therapeutic development for cystic fibrosis. Int. J. Mol. Sci. 21, 836 (2020).
Viart, V. et al. Transcription factors and miRNAs that regulate fetal to adult CFTR expression change are new targets for cystic fibrosis. Eur. Respir. J. 45, 116–128 (2015).
Sonneville, F. et al. MicroRNA-9 downregulates the ANO1 chloride channel and contributes to cystic fibrosis lung pathology. Nat. Commun. 8, 1–11 (2017).
Aránega, A. E. et al. MiRNAs and muscle regeneration: therapeutic targets in Duchenne muscular dystrophy. Int. J. Mol. Sci. 22, 4236 (2021).
Odom, G. L., Gregorevic, P. & Chamberlain, J. S. Viral-mediated gene therapy for the muscular dystrophies: successes, limitations and recent advances. Biochim. Biophys. Acta 1772, 243 (2007).
Wang, L. et al. Loss of miR-29 in myoblasts contributes to dystrophic muscle pathogenesis. Mol. Ther. 20, 1222 (2012).
Heller, K. N., Mendell, J. T., Mendell, J. R. & Rodino-Klapac, L. R. MicroRNA-29 overexpression by adeno-associated virus suppresses fibrosis and restores muscle function in combination with micro-dystrophin. JCI Insight 2, e93309 (2017).
Cacchiarelli, D. et al. miR-31 modulates dystrophin expression: new implications for Duchenne muscular dystrophy therapy. EMBO Rep. 12, 136–141 (2011).
Bader, A. G., Brown, D. & Winkler, M. The promise of microRNA replacement therapy. Cancer Res. 70, 7027–7030 (2010).
Gruszka, R., Zakrzewski, K., Liberski, P. P. & Zakrzewska, M. mRNA and miRNA expression analyses of the MYC/E2F/miR-17-92 network in the most common pediatric brain tumors. Int. J. Mol. Sci. 22, 1–14 (2021).
Kurkewich, J. L. et al. The mirn23a and mirn23b microRNA clusters are necessary for proper hematopoietic progenitor cell production and differentiation. Exp. Hematol. 59, 14 (2018).
Battistella, M. & Marsden, P. A. Advances, nuances, and potential pitfalls when exploiting the therapeutic potential of RNA interference. Clin. Pharmacol. Ther. 97, 79–87 (2015).
Bernardo, B. C., Ooi, J. Y. Y., Lin, R. C. Y. & Mcmullen, J. R. miRNA therapeutics: a new class of drugs with potential therapeutic applications in the heart. Future Med. Chem. 7, 1771–1792 (2015).
Castanotto, D. & Rossi, J. J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 457, 426 (2009).
Murphy, D. E. et al. Natural or synthetic RNA delivery: a stoichiometric comparison of extracellular vesicles and synthetic nanoparticles. Nano Lett. 21, 1888–1895 (2021).
Hayes, J., Peruzzi, P. P. & Lawler, S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol. Med. 20, 460–469 (2014).
Feng, M. et al. Macrophages eat cancer cells using their own calreticulin as a guide: roles of TLR and Btk. Proc. Natl Acad. Sci. USA 112, 2145–2150 (2015).
Hanna, J., Hossain, G. S. & Kocerha, J. The potential for microRNA therapeutics and clinical research. Front. Genet. 10, 478 (2019).
Elsharkasy, O. M. et al. Extracellular vesicles as drug delivery systems: why and how? Adv. Drug Deliv. Rev. 159, 332–343 (2020).
Binderup, H. G. et al. Quantification of microRNA levels in plasma – impact of preanalytical and analytical conditions. PLoS ONE 13, e0201069 (2018).
Ziemann, M., Kaspi, A. & El-Osta, A. Evaluation of microRNA alignment techniques. RNA 22, 1120–1138 (2016).
Faraldi, M. et al. Normalization strategies differently affect circulating miRNA profile associated with the training status. Sci. Rep. 9, 1–13 (2019).
Zhao, H. et al. Identification of valid reference genes for mRNA and microRNA normalisation in prostate cancer cell lines. Sci. Rep. 8, 1949 (2018).
Serafin, A. et al. Identification of a set of endogenous reference genes for miRNA expression studies in Parkinson’s disease blood samples. BMC Res. Notes 7, 1–9 (2014).
Zhang, Y., Tang, W., Peng, L., Tang, J. & Yuan, Z. Identification and validation of microRNAs as endogenous controls for quantitative polymerase chain reaction in plasma for stable coronary artery disease. Cardiol. J. 23, 694–703 (2016).
Hicks, S. D. et al. Diurnal oscillations in human salivary microRNA and microbial transcription: Implications for human health and disease. PLoS ONE 13, e0198288 (2018).
Fehlmann, T. et al. Common diseases alter the physiological age-related blood microRNA profile. Nat. Commun. 11, 5958 (2020).
Hicks, S. D., Jacob, P., Middleton, F. A., Perez, O. & Gagnon, Z. Distance running alters peripheral microRNAs implicated in metabolism, fluid balance, and myosin regulation in a sex-specific manner. Physiol. Genomics 50, 658–667 (2018).
Scipioni, A. M., Ornstein, R. M. & Hicks, S. D. Differential expression of salivary microRNA in anorexia nervosa and anxiety disorders. J. Adolesc. Health 64, S13 (2019).
Kinoshita, C., Okamoto, Y., Aoyama, K. & Nakaki, T. MicroRNA: a key player for the interplay of circadian rhythm abnormalities, sleep disorders and neurodegenerative diseases. Clocks Sleep 2, 282 (2020).
Funding
No financial assistance was received in support of this work. SDH’s time was supported by the National Institutes of Health (grant numbers R61HD105610, R01NS115942, and 4R61HD105610-02 to S.D.H.) and the Gerber Foundation (grant number 5295 to S.D.H.).
Author information
Authors and Affiliations
Contributions
S.D.H., C.L., and R.E.S. had substantial contributions to drafting the manuscript. R.E.S. revised the manuscript critically for important intellectual content. S.D.H. and D.Z. gave the final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
S.D.H. serves as a scientific advisory board member for Quadrant Biosciences, a biotech company involved in the development of RNA-based diagnostics. Quadrant Biosciences had no role in drafting or editing the proposed review article. S.D.H. is a paid consultant and scientific advisory board member for Quadrant Biosciences. He is named as a co-inventor on intellectual property involving salivary microRNA biomarkers, which has been patented by the Penn State College of Medicine. These entities had no role in the design, analysis, or writing of this manuscript and played no part in the decision to submit this article for publication. All other authors have no conflicts to report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, C., Sullivan, R.E., Zhu, D. et al. Putting the “mi” in omics: discovering miRNA biomarkers for pediatric precision care. Pediatr Res 93, 316–323 (2023). https://doi.org/10.1038/s41390-022-02206-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02206-5