Abstract
Background
Treatment of neonatal peritonitis and sepsis is challenging. Following infection, neutrophils elaborate neutrophil extracellular traps (NETs)—extracellular lattices of decondensed chromatin decorated with antimicrobial proteins. NETs, however, can augment pathogenic inflammation causing collateral damage. We hypothesized that NET inhibition would improve survival in experimental neonatal infectious peritonitis.
Methods
We induced peritonitis in 7 to 10-day-old mice by intraperitoneal injection with cecal slurry. We targeted NETs by treating mice with neonatal NET-Inhibitory Factor (nNIF), an endogenous NET-inhibitor; Cl-amidine, a PAD4 inhibitor; DNase I, a NET degrading enzyme, or meropenem (an antibiotic). We determined peritoneal NET and cytokine levels and circulating platelet–neutrophil aggregates. Survival from peritonitis was followed for 6 days.
Results
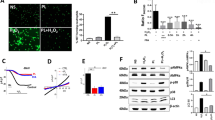
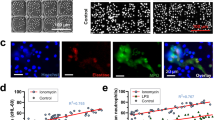
nNIF, Cl-amidine, and DNase I decreased peritoneal NET formation and inflammatory cytokine levels at 24 h compared to controls. nNIF, Cl-amidine, and DNase I decreased circulating platelet–neutrophil aggregates, and NET-targeting treatments significantly increased survival from infectious peritonitis compared to controls. Finally, nNIF administration significantly improved survival in mice treated with sub-optimal doses of meropenem even when treatment was delayed until 2 h after peritonitis induction.
Conclusions
NET inhibition improves survival in experimental neonatal infectious peritonitis, suggesting that NETs participate pathogenically in neonatal peritonitis and sepsis.
Impact
-
1.
Neutrophil extracellular trap formation participates pathogenically in experimental neonatal infectious peritonitis.
-
2.
NET-targeting strategies improve outcomes in a translational model of neonatal infectious peritonitis.
-
3.
NET inhibition represents a potential target for drug development in neonatal sepsis and infectious peritonitis.

Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Reinhart, K. et al. Recognizing sepsis as a global health priority—a WHO resolution. N. Engl. J. Med. 377, 414–417 (2017).
Fleischmann-Struzek, C. et al. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir. Med. 6, 223–230 (2018).
Shane, A. L., Sánchez, P. J. & Stoll, B. J. Neonatal sepsis. Lancet 390, 1770–1780 (2017).
Weston, E. J. et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005-2008. Pediatr. Infect. Dis. J. 30, 937–941 (2011).
Seale, A. C. et al. Estimates of possible severe bacterial infection in neonates in sub-Saharan Africa, south Asia, and Latin America for 2012: a systematic review and meta-analysis. Lancet Infect. Dis. 14, 731–741 (2014).
Kermorvant-Duchemin, E., Laborie, S., Rabilloud, M., Lapillonne, A. & Claris, O. Outcome and prognostic factors in neonates with septic shock. Pediatr. Crit. Care Med. 9, 186–191 (2008).
Wynn, J. L. & Wong, H. R. Pathophysiology and treatment of septic shock in neonates. Clin. Perinatol. 37, 439–479 (2010).
Cortese, F. et al. Early and late infections in newborns: where do we stand? A review. Pediatr. Neonatol. 57, 265–273 (2016).
Shane, A. L. & Stoll, B. J. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. Am. J. Perinatol. 30, 131–141 (2013).
Schüller, S. S. et al. Immunomodulation to prevent or treat neonatal sepsis: past, present, and future. Front. Pediatr. 6, 199 (2018).
Rittirsch, D., Flierl, M. A. & Ward, P. A. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 8, 776–787 (2008).
Castanheira, F. V. S. & Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 133, 2178–2185 (2019).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Sorvillo, N., Cherpokova, D., Martinod, K. & Wagner, D. D. Extracellular DNA NET-works with dire consequences for health. Circ. Res. 125, 470–488 (2019).
Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 (2018).
Wynn, J., Cornell, T. T., Wong, H. R., Shanley, T. P. & Wheeler, D. S. The host response to sepsis and developmental impact. Pediatrics 125, 1031–1041 (2010).
Zhang, X., Zhivaki, D. & Lo-Man, R. Unique aspects of the perinatal immune system. Nat. Rev. Immunol. 17, 495–507 (2017).
Yost, C. C. et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood 113, 6419–6427 (2009).
Zonneveld, R. et al. Soluble adhesion molecules as markers for sepsis and the potential pathophysiological discrepancy in neonates, children and adults. Crit. Care 18, 204 (2014).
Wynn, J. L. et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 28, 675–683 (2007).
Starr, M. E. et al. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS ONE 9, e115705 (2014).
Campbell, R. A. et al. Placental HTRA1 cleaves α1-antitrypsin to generate a NET-inhibitory peptide. Blood 138, 977–988 (2021).
Yost, C. C. et al. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. J. Clin. Invest. 126, 3783–3798 (2016).
Middleton, E. A. et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 136, 1169–1179 (2020).
Denorme, F. et al. Platelet necrosis mediates ischemic stroke outcome in mice. Blood 135, 429–440 (2020).
Denorme, F., Portier, I., Kosaka, Y. & Campbell, R. A. Hyperglycemia exacerbates ischemic stroke outcome independent of platelet glucose uptake. J. Thromb. Haemost. 19, 536–546 (2021).
Guo, L. & Rondina, M. T. The era of thromboinflammation: platelets are dynamic sensors and effector cells during infectious diseases. Front. Immunol. 10, 2204 (2019).
Denorme, F., Rustad, J. L. & Campbell, R. A. Brothers in arms: platelets and neutrophils in ischemic stroke. Curr. Opin. Hematol. https://doi.org/10.1097/MOH.0000000000000665 (2021).
Denorme, F. et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J. Clin. Invest. https://doi.org/10.1172/JCI154225 (2022).
Trinchieri, G. & Sher, A. Cooperation of toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7, 179–190 (2007).
Wei, Y., Kim, J., Ernits, H. & Remick, D. The septic neutrophil-friend or foe. Shock 55, 147–155 (2021).
Ye, Q., Du, L.-Z., Shao, W.-X. & Shang, S.-Q. Utility of cytokines to predict neonatal sepsis. Pediatr. Res. 81, 616–621 (2017).
Hakkim, A. et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA 107, 9813–9818 (2010).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Czaikoski, P. G. et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS ONE 11, e0148142 (2016).
Yang, S. et al. Neutrophil extracellular traps promote hypercoagulability in patients with sepsis. Shock 47, 132–139 (2017).
Hoppenbrouwers, T. et al. Neutrophil extracellular traps in children with meningococcal sepsis. Pediatr. Crit. Care Med. 19, e286–e291 (2018).
Colón, D. F. et al. Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit. Care 23, 113 (2019).
Zuo, Y. et al. Neutrophil extracellular traps in COVID-19. JCI Insight 5, e138999 (2020).
Nicolai, L. et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation 142, 1176–1189 (2020).
Biron, B. M. et al. Cl-amidine prevents histone 3 citrullination and neutrophil extracellular trap formation, and improves survival in a murine sepsis model. J. Innate Immun. 9, 22–32 (2017).
Biron, B. M. et al. PAD4 deficiency leads to decreased organ dysfunction and improved survival in a dual insult model of hemorrhagic shock and sepsis. J. Immunol. 200, 1817–1828 (2018).
Hawez, A. et al. MiR-155 regulates neutrophil extracellular trap formation and lung injury in abdominal sepsis. J. Leukoc. Biol. https://doi.org/10.1002/JLB.3A1220-789RR (2021).
Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 (2007).
Sreeramkumar, V. et al. Neutrophils scan for activated platelets to initiate inflammation. Science (1979) 346, 1234–1238 (2014).
Jorch, S. K. & Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 23, 279–287 (2017).
Massberg, S. et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 16, 887–896 (2010).
Stark, K. & Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. https://doi.org/10.1038/s41569-021-00552-1 (2021).
Knight, J. S. et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J. Clin. Invest. 123, 2981–2993 (2013).
Fuchs, T. A. et al. Extracellular DNA traps promote thrombosis. Proc. Natl Acad. Sci. USA 107, 15880–15885 (2010).
Lauková, L. et al. Exogenous deoxyribonuclease has a protective effect in a mouse model of sepsis. Biomed. Pharmacother. 93, 8–16 (2017).
Mai, S. H. C. et al. Delayed but not early treatment with dnase reduces organ damage and improves outcome in a murine model of sepsis. Shock 44, 166–172 (2015).
Meng, W. et al. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit. Care 16, R137 (2012).
Yipp, B. G. et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18, 1386–1393 (2012).
Menegazzi, R., Decleva, E. & Dri, P. Killing by neutrophil extracellular traps: fact or folklore? Blood 119, 1214–1216 (2012).
McDonald, B. et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129, 1357–1367 (2017).
Carestia, A., Davis, R. P., Davis, L. & Jenne, C. N. Inhibition of immunothrombosis does not affect pathogen capture and does not promote bacterial dissemination in a mouse model of sepsis. Platelets 31, 925–931 (2020).
McDonald, B., Urrutia, R., Yipp, B. G., Jenne, C. N. & Kubes, P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 12, 324–333 (2012).
Li, P. et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207, 1853–1862 (2010).
Funding
This work was supported in part by the US NIH (R01HD093826 to C.C.Y.—NICHD; K01AG059892 to R.A.C.—NIA), PEEL Therapeutics, Inc. (Sponsored Research Agreement to C.C.Y.), the American Heart Association (2021Post830138 to F.D. and 2022Post906231 to I.P.), and by the University of Utah Department of Pediatrics, Division of Neonatology.
Author information
Authors and Affiliations
Contributions
C.C.Y. and F.D. conceived the project and designed the experiments. F.D., J.L.R., I.P., J.L.C., C.V.d.A., and M.J.C. performed experiments and analyzed data. F.D., M.J.C., R.A.C., and C.C.Y. wrote and edited the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
C.C.Y. authors a US patent (patent 232,023 B2) held by the University of Utah for the use of NET-inhibitory peptides for the “treatment of and prophylaxis against inflammatory disorders,” for which PEEL Therapeutics, Inc. holds the exclusive license. The other authors declare that no additional conflict of interest exists.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Denorme, F., Rustad, J.L., Portier, I. et al. Neutrophil extracellular trap inhibition improves survival in neonatal mouse infectious peritonitis. Pediatr Res 93, 862–869 (2023). https://doi.org/10.1038/s41390-022-02219-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02219-0