Abstract
Background
Urinary tract obstruction is associated with impaired renal urinary concentration; even after the release of the obstruction, patients still suffer from polyuria. It has been reported that the decreased expression of aquaporins (AQPs) is associated with postobstructive polyuria, and erythropoietin (EPO) can promote the recovery of decreased AQP2 expression induced by bilateral ureteral obstruction. However, whether EPO can promote the recovery of the expression of AQP1–3 after the release of unilateral ureteral obstruction (UUO) has not yet been reported.
Aims
To investigate the effects of EPO treatment on the expression of renal AQP1–3 after the release of UUO.
Methods
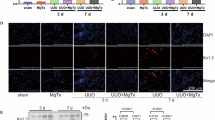
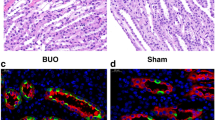
UUO was established in rats by 24-h temporary unilateral obstruction of renal ureters. Three days following EPO treatment, the kidneys were removed to determine the expression levels of AQP1–3, NLRP3, caspase-1, and IL-1β via semiquantitative immunoblotting and immunohistochemistry.
Results
EPO inhibited the expression of NLRP3, caspase-1, and IL-1β; reduced plasma creatinine and urea; and promoted the recovery of AQP1–3 expression in UUO rats.
Conclusions
EPO treatment prevented the decreased expression of renal AQPs and the development of impaired urinary concentration capacity after the release of UUO, which may partially occur by way of anti-inflammasome effects.
Impact
-
EPO treatment could prevent the decreased expression of renal water transporter proteins AQP1–3 and the development of impaired renal functions, which may be associated with its anti-inflammasome effects.
-
EPO regulated the expression of renal water transporter proteins AQP1–3, which could provide the potential for the treatment of postobstructive polyuresis.
-
EPO treatment could be one of the effective methods by participating in multiple dimensions for patients with obstructive nephropathy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Trnka, P., Hiatt, M. J., Tarantal., A. F. & Matsell, D. G. Congenital urinary tract obstruction: defining markers of developmental kidney injury. Pediatr. Res. 72, 446–454 (2012).
Li, Z. Z. et al. Decrease of renal aquaporins 1-4 is associated with renal function impairment in pediatric congenital hydronephrosis. World J. Pediatr. 8, 335–341 (2012).
Harris, K. P., Schreiner, G. F. & Klahr, S. Effect of leukocyte depletion on the function of the postobstructed kidney in the rat. Kidney Int. 36, 210–215 (1989).
Ricardo, S. D. & Diamond, J. R. The role of macrophages and reactive oxygen species in experimental hydronephrosis. Semin Nephrol. 18, 612–621 (1998).
Li, C. et al. Downregulation of renal aquaporins in response to unilateral ureteral obstruction. Am. J. Physiol. Ren. Physiol. 284, 1066–1079 (2003).
Li, C. et al. alpha-MSH prevents impairment in renal function and dysregulation of AQPs and Na-K-ATPase in rats with bilateral ureteral obstruction. Am. J. Physiol. Ren. Physiol. 290, F384–F396 (2006).
Li, C. et al. Downregulation of AQP1, −2, and −3 after ureteral obstruction is associated with a long-term urine-concentrating defect. Am. J. Physiol. Ren. Physiol. 281, F163–F171 (2001).
Komada, T. & Muruve, D. A. The role of inflammasomes in kidney disease. Nat. Rev. Nephrol. 15, 501–520 (2019).
Davis, B. K., Wen, H. & Ting, J. P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev. Immunol. 29, 707–735 (2011).
Wu, M. et al. NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol. Cell Endocrinol. 478, 115–125 (2018).
Gong, W. et al. NLRP3 deletion protects against renal fibrosis and attenuates mitochondrial abnormality in mouse with 5/6 nephrectomy. Am. J. Physiol. Ren. Physiol. 310, F1081–F1088 (2016).
Ludwig-Portugall, I. et al. An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int. 90, 525–539 (2016).
Chen, Y. et al. Forskolin attenuates the NLRP3 inflammasome activation and IL-1β secretion in human macrophages. Pediatr. Res. 86, 692–698 (2019).
Zhang, Y., Yang, W., Li, W. & Zhao, Y. NLRP3 inflammasome: checkpoint connecting innate and adaptive immunity in autoimmune diseases. Front. Immunol. 12, 732933 (2021).
Peng, B., Kong, G., Yang, C. & Ming, Y. Erythropoietin and its derivatives: from tissue protection to immune regulation. Cell Death Dis. 11, 79 (2020).
Ren, C. C. et al. The renal protect function of erythropoietin after release of bilateral ureteral obstruction in a rat model. Clin. Sci. (Lond.). 132, 2071–2085 (2018).
Gong, H. et al. EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney. Kidney Int. 66, 683–695 (2004).
Yang, C. W. et al. Preconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in rat kidney. FASEB J. 17, 1754–1755 (2003).
Kitamura, H. et al. Nonerythropoietic derivative of erythropoietin protects against tubulointerstitial injury in a unilateral ureteral obstruction model. Nephrol. Dial. Transplant. 23, 1521–1528 (2008).
Trnka, P., Hiatt, M. J., Tarantal, A. F. & Matsell, D. G. Congenital urinary tract obstruction: defining markers of developmental kidney injury. Pediatr. Res. 72, 446–454 (2012).
Ding, B., Ma, G., Wang, Z., Liang, W. & Gao, W. Mechanisms of kidney cell pyroptosis in chronic kidney disease and the effects of traditional chinese medicine. Evid. Based Complement Altern. Med. 2021, 1173324 (2021).
Hsu, W. H. et al. Compound K inhibits priming and mitochondria-associated activating signals of NLRP3 inflammasome inrenal tubulointerstitial lesions. Nephrol. Dial. Transplant. 35, 74–85 (2020).
Schreiner, G. F., Harris, K. P., Purkerson, M. L. & Klahr, S. Immunological aspects of acute ureteral obstruction: immune cell infiltrate in the kidney. Kidney Int. 34, 487–493 (1988).
Yeh, C. H., Chiang, H. S., Lai, T. Y. & Chien, C. T. Unilateral ureteral obstruction evokes renal tubular apoptosis via the enhanced oxidative stress and endoplasmic reticulum stress in the rat. Neurourol. Urodyn. 30, 472–479 (2011).
Kim, Y. G. et al. The role of inflammasome-dependent and inflammasome-independent NLRP3 in the kidney. Cells 8, 1389 (2019).
Su, W., Cao, R., Zhang, X. Y. & Guan, Y. Aquaporins in the kidney: physiology and pathophysiology. Am. J. Physiol. Ren. Physiol. 318, F193–F203 (2020).
Wen, J. G. et al. Expression of renal aquaporins is down-regulated in children with congenital hydronephrosis. Scand. J. Urol. Nephrol. 43, 486–493 (2009).
Feng, J. J. et al. Aquaporin1-3 expression in normal and hydronephrotic kidneys in the human fetus. Pediatr. Res. 86, 595–602 (2019).
Boesen, E. I. Chronic elevation of IL-1β induces diuresis via a cyclooxygenase 2-mediated mechanism. Am. J. Physiol. Ren. Physiol. 305, F189–F198 (2013).
Boesen, E. I. et al. Interleukin-1beta, but not interleukin-6, enhances renal and systemic endothelin production in vivo. Am. J. Physiol. Ren. Physiol. 295, F446–F453 (2008).
Wang, W. et al. Aliskiren restores renal AQP2 expression during unilateral ureteral obstruction by inhibiting the inflammasome. Am. J. Physiol. Ren. Physiol. 308, F910–F922 (2015).
Zhang, J. et al. Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin II-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab. 23, 360–368 (2016).
Stoos, B. A., Garcia, N. H. & Garvin, J. L. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J. Am. Soc. Nephrol. 6, 89–94 (1995).
García, N. H., Pomposiello, S. I. & Garvin, J. L. Nitric oxide inhibits ADH-stimulated osmotic water permeability in cortical collecting ducts. Am. J. Physiol. 270, F206–F210 (1996).
Komada, T. et al. ASC in renal collecting duct epithelial cells contributes to inflammation and injury after unilateral ureteral obstruction. Am. J. Pathol. 184, 1287–1298 (2014).
Acknowledgements
We would like to thank Keke Ma, who was the administrator of the Laboratory Animal Center of Henan Province for helping us to feed the animals.
Funding
This study was funded by the Medical Science and Technology Research-related joint construction project of Henan Province (7220) and the Natural Science Foundation of China (No. U1904208).
Author information
Authors and Affiliations
Contributions
J.F., J.W., Y.Z., B.D., J.T., S.Yu, S.Yan, E.L., L.L., and X.Z. made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. J.F., J.W., B.D., Y.Z., J.T., S.Yu, and S.Yan made substantial contributions to make animal model. J.F., X.Z., J.W., Y.Z., and B.D. drafted the article or revised it critically for important intellectual content. X.Z. made the final approval of the version to be published. J.F., J.W., and Y.Z. made equal contribution to the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures conformed to the Chinese National Guidelines for the Care and Handling of Animals and the published guidelines from the National Institutes of Clinical Medicine, Zhengzhou University, according to the licenses for use of experimental animals issued by the Chinese Ministry of Justice (2020-KY-273).
Consent for publication
The submission of this manuscript is approved by all authors for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, J., Wen, J., Zhang, Y. et al. Erythropoietin prevented the decreased expression of aquaporin1–3 in ureteral obstructive kidneys in juvenile rats. Pediatr Res 93, 1258–1266 (2023). https://doi.org/10.1038/s41390-022-02224-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02224-3