Abstract
Background
The accumulation of short-chain fatty acids (SCFAs) from bacterial fermentation may adversely affect the under-developed gut as observed in premature newborns at risk for necrotizing enterocolitis (NEC). This study explores the mechanism by which specific SCFA fermentation products may injure the premature newborn intestine mucosa leading to NEC-like intestinal cell injury.
Methods
Intraluminal injections of sodium butyrate were administered to 14- and 28-day-old mice, whose small intestine and stool were harvested for analysis. Human intestinal epithelial stem cells (hIESCs) and differentiated enterocytes from preterm and term infants were treated with sodium butyrate at varying concentrations. Necrosulfonamide (NSA) and necrostatin-1 (Nec-1) were used to determine the protective effects of necroptosis inhibitors on butyrate-induced cell injury.
Results
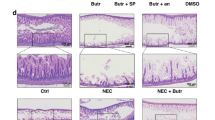
The more severe intestinal epithelial injury was observed in younger mice upon exposure to butyrate (p = 0.02). Enterocytes from preterm newborns demonstrated a significant increase in sensitivity to butyrate-induced cell injury compared to term newborn enterocytes (p = 0.068, hIESCs; p = 0.038, differentiated cells). NSA and Nec-1 significantly inhibited the cell death induced by butyrate.
Conclusions
Butyrate induces developmental stage-dependent intestinal injury that resembles NEC. A primary mechanism of cell injury in NEC is necroptosis. Necroptosis inhibition may represent a potential preventive or therapeutic strategy for NEC.
Impact
-
Butyrate induces developmental stage-dependent intestinal injury that resembles NEC.
-
A primary mechanism of cell injury caused by butyrate in NEC is necroptosis.
-
Necroptosis inhibitors proved effective at significantly ameliorating the enteral toxicity of butyrate and thereby suggest a novel mechanism and approach to the prevention and treatment of NEC in premature newborns.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Nathan, A. T. et al. A quality improvement initiative to reduce necrotizing enterocolitis across hospital systems. J. Perinatol. 38, 742–750 (2018).
Elgin, T. G., Kern, S. L. & McElroy, S. J. Development of the neonatal intestinal microbiome and its association with necrotizing enterocolitis. Clin. Ther. 38, 706–715 (2016).
Eaton, S., Rees, C. M. & Hall, N. J. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology 111, 423–430 (2017).
Denning, T. L. et al. Pathogenesis of NEC: role of the innate and adaptive immune response. Semin. Perinatol. 41, 15–28 (2017).
Neu, J. & Pammi, M. Pathogenesis of NEC: impact of an altered intestinal microbiome. Semin. Perinatol. 41, 29–35 (2017).
Warner, B. B. et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 387, 1928–1936 (2016).
Lin, J. et al. Short-chain fatty acid induces intestinal mucosal injury in newborn rats and down-regulates intestinal trefoil factor gene expression in vivo and in vitro. J. Pediatr. Gastroenterol. Nutr. 41, 607–611 (2005).
den Besten, G. et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340 (2013).
Jiang, P. et al. Premature delivery reduces intestinal cytoskeleton, metabolism, and stress response proteins in newborn formula-fed pigs. J. Pediatr. Gastroenterol. Nutr. 56, 615–622 (2013).
Sylvester, K. G. et al. Acylcarnitine profiles reflect metabolic vulnerability for necrotizing enterocolitis in newborns born premature. J. Pediatr. 181, 80–85.e1 (2017).
Szylit, O. et al. Fecal short-chain fatty acids predict digestive disorders in premature infants. Jpen. J. Parenter. Enter. Nutr. 22, 136–141 (1998).
Kaiko, G. E. et al. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165, 1708–1720 (2016).
Thymann, T. et al. Carbohydrate maldigestion induces necrotizing enterocolitis in preterm pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G1115–G1125 (2009).
Nafday, S. M. et al. Short-chain fatty acids induce colonic mucosal injury in rats with various postnatal ages. Pediatr. Res. 57, 201–204 (2005).
Garg, P. M. et al. Necrotizing enterocolitis in a mouse model leads to widespread renal inflammation, acute kidney injury, and disruption of renal tight junction proteins. Pediatr. Res. 78, 527–532 (2015).
McElroy, S. J. et al. The ErbB4 ligand neuregulin-4 protects against experimental necrotizing enterocolitis. Am. J. Pathol. 184, 2768–2778 (2014).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 (2011).
VanDussen, K. L. et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64, 911–920 (2015).
Sato, T. & Clevers, H. Primary mouse small intestinal epithelial cell cultures. Methods Mol. Biol. 945, 319–328 (2013).
In, J. et al. Enterohemorrhagic Escherichia coli reduce mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell Mol. Gastroenterol. Hepatol. 2, 48–62.e3 (2016).
Lu, P. et al. Animal models of gastrointestinal and liver diseases. Animal models of necrotizing enterocolitis: pathophysiology, translational relevance, and challenges. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G917–G928 (2014).
Xing, T., Camacho Salazar, R. & Chen, Y. H. Animal models for studying epithelial barriers in neonatal necrotizing enterocolitis, inflammatory bowel disease and colorectal cancer. Tissue Barriers 5, e1356901 (2017).
Polari, L. et al. Keratin intermediate filaments in the colon: guardians of epithelial homeostasis. Int. J. Biochem. Cell Biol. 129, 105878 (2020).
Fitzgibbons, S. C. et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg. 44, 1072–1075 (2009). discussion 1075-1076.
Patel, R. M. et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N. Engl. J. Med. 372, 331–340 (2015).
Mericq, V. et al. Long-term metabolic risk among children born premature or small for gestational age. Nat. Rev. Endocrinol. 13, 50–62 (2017).
Kim, M. et al. Immature oxidative stress management as a unifying principle in the pathogenesis of necrotizing enterocolitis: insights from an agent-based model. Surg. Infect. 13, 18–32 (2012).
Pourcyrous, M. et al. Fecal short-chain fatty acids of very-low-birth-weight preterm infants fed expressed breast milk or formula. J. Pediatr. Gastroenterol. Nutr. 59, 725–731 (2014).
Hung, T. V. & Suzuki, T. Short-chain fatty acids suppress inflammatory reactions in Caco-2 cells and mouse colons. J. Agric. Food Chem. 66, 108–117 (2018).
Chen, G. et al. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine 30, 317–325 (2018).
Qiu, Y. et al. Effect of sodium butyrate on cell proliferation and cell cycle in porcine intestinal epithelial (IPEC-J2) cells. Vitr. Cell. Dev. Biol. Anim. 53, 304–311 (2017).
Mirpuri, J. et al. Commensal Escherichia coli reduces epithelial apoptosis through IFN-alphaA-mediated induction of guanylate binding protein-1 in human and murine models of developing intestine. J. Immunol. 184, 7186–7195 (2010).
Lin, P. W. et al. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic. Biol. Med. 47, 1205–1211 (2009).
Wen, S. et al. Necroptosis is a key mediator of enterocytes loss in intestinal ischaemia/reperfusion injury. J. Cell. Mol. Med. 21, 432–443 (2017).
Zhang, T. et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat. Med. 22, 175–182 (2016).
Newton, K. et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343, 1357–1360 (2014).
Quarato, G. et al. Sequential engagement of distinct MLKL phosphatidylinositol-binding sites executes necroptosis. Mol. Cell 61, 589–601 (2016).
Pierdomenico, M. et al. Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am. J. Gastroenterol. 109, 279–287 (2014).
Werts, A. D. et al. A novel role for necroptosis in the pathogenesis of necrotizing enterocolitis. Cell Mol. Gastroenterol. Hepatol. 9, 403–423 (2020).
Li, X. et al. MiR-141-3p ameliorates RIPK1-mediated necroptosis of intestinal epithelial cells in necrotizing enterocolitis. Aging 12, 18073–18083 (2020).
Liu, T. et al. Toll-like receptor 4-mediated necroptosis in the development of necrotizing enterocolitis. Pediatr. Res. 91, 73–82 (2022).
Dong, W. et al. Protective effect of NSA on intestinal epithelial cells in a necroptosis model. Oncotarget 8, 86726–86735 (2017).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Acknowledgements
We are grateful to Dr. Teri A. Longacre for acquisition of IHC images.
Author information
Authors and Affiliations
Contributions
K.G.S. and J.D. conceived the study. K.W. and G.-Z.T. contributed to the study design. K.W. drafted the manuscript. K.W., G.-Z.T., P.-Y.L., Z.S., B.L., and M.M. contributed to experiments and data acquisition. K.W., G.-Z.T., P.-Y.L., F.S.-J., T.S., J.D., and K.G.S. contributed to data analysis. F.S.-J. and K.G.S. edited the draft and contributed to the final submitted version. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Caretakers of all the participants had signed written informed consent before participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, K., Tao, GZ., Salimi-Jazi, F. et al. Butyrate induces development-dependent necrotizing enterocolitis-like intestinal epithelial injury via necroptosis. Pediatr Res 93, 801–809 (2023). https://doi.org/10.1038/s41390-022-02333-z
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02333-z
This article is cited by
-
Short-chain fatty acids—a key link between the gut microbiome and T-lymphocytes in neonates?
Pediatric Research (2025)
-
Receptor-interacting protein kinase-3 (RIPK3): a new biomarker for necrotising enterocolitis in preterm infants
Pediatric Surgery International (2024)