Abstract
Background
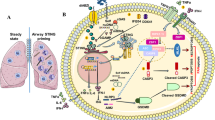
The pathogenesis of congenital diaphragmatic hernia (CDH) depends on multiple factors. Activation of the DNA-sensing cyclic-GMP-AMP-synthase (cGAS) and Stimulator-of-Interferon-Genes (STING) pathway by double-stranded DNA (dsDNA) links environmental stimuli and inflammation. We hypothesized that nitrofen exposure alters cGAS and STING in human bronchial epithelial cells and fetal rat lungs.
Methods
We used the Quant-IT™-PicoGreen™ assay to assess dsDNA concentration in BEAS-2B cells after 24 h of nitrofen-exposure and performed immunofluorescence of cGAS/STING. We used nitrofen to induce CDH and harvested control and CDH lungs at embryonic day E15, E18 and E21 for cGAS/STING immunofluorescence, RT-qPCR and RNA-Scope™ in-situ-hybridization (E18, E21).
Results
We found a higher concentration of dsDNA following nitrofen treatment. Nitrofen-exposure to BEAS-2B cells increased cGAS and STING protein abundance. cGAS abundance was higher in nitrofen lungs at E15, E18 and E21. RNA-Scope in-situ-hybridization showed higher cGAS and STING expression in E18 and E21 lungs. RT-qPCR revealed higher mRNA expression levels of STING in E21 nitrofen-induced lungs.
Conclusion
Our data suggest that nitrofen-exposure increases dsDNA content which leads to stimulation of the cGAS/STING pathway in human BEAS-2B cells and the nitrofen rat model of CDH. Consequently, DNA sensing and the cGAS-STING-pathway potentially contribute to abnormal lung development in CDH.
Impact statement
We found an alteration of DNA sensing targets cGAS and STING in human BEAS-2B cells and experimental congenital diaphragmatic hernia with higher protein abundance and mRNA expression in cells and lung sections of nitrofen-treated rat pups. This is the first study to investigate DNA sensing, a potential link between environmental stimuli and inflammation, in experimental CDH. Our study extends the knowledge on the pathogenesis of experimental CDH.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Zani, A. et al. Congenital diaphragmatic hernia. Nat. Rev. Dis. Prim. 8, 37 (2022).
Yu, L., Hernan, R. R., Wynn, J. & Chung, W. K. The influence of genetics in congenital diaphragmatic hernia. Semin. Perinatol. 44, 1–19 (2020).
Ito, Y. et al. Vitamin D improves pulmonary function in a rat model for congenital diaphragmatic hernia. Arch. Biochem. Biophys. 700, 108769 (2021).
Michikawa, T. et al. Maternal dietary intake of vitamin A during pregnancy was inversely associated with congenital diaphragmatic hernia: the Japan Environment and Children’s Study. Br. J. Nutr. 122, 1295–1302 (2019).
Wagner, R. et al. Proteomic profiling of hypoplastic lungs suggests an underlying inflammatory response in the pathogenesis of abnormal lung development in congenital diaphragmatic hernia. Ann. Surg. https://doi.org/10.1097/SLA.0000000000005656 (2022).
Antounians, L. et al. Administration of amniotic fluid stem cell extracellular vesicles promotes development of fetal hypoplastic lungs by immunomodulating lung macrophages. bioRxiv 2022.11.29.518388 https://doi.org/10.1101/2022.11.29.518388 (2022).
Wagner, R. et al. A tracheal aspirate-derived airway basal cell model reveals a proinflammatory epithelial defect in congenital diaphragmatic hernia. Am. J. Respir. Crit. Care Med. https://doi.org/10.1164/rccm.202205-0953OC (2023).
Auriti, C. et al. Pregnancy and viral infections: Mechanisms of fetal damage, diagnosis and prevention of neonatal adverse outcomes from cytomegalovirus to SARS-CoV-2 and Zika virus. Biochim. Biophys. acta Mol. basis Dis. 1867, 166198 (2021).
James, S. H. & Kimberlin, D. W. Neonatal Herpes Simplex Virus Infection. Infect. Dis. Clin. North Am. 29, 391–400 (2015).
Akhtar, J. & Shukla, D. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 276, 7228–7236 (2009).
Motwani, M., Pesiridis, S. & Fitzgerald, K. A. DNA sensing by the cGAS–STING pathway in health and disease. Nat. Rev. Genet. 20, 657–674 (2019).
Kluth, D. et al. Nitrofen-induced diaphragmatic hernias in rats: An animal model. J. Pediatr. Surg. 25, 850–854 (1990).
Arganda-Carreras, I. et al. Trainable Weka Segmentation: a machine learning tool for microscopy pixel classification. Bioinformatics 33, 2424–2426 (2017).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Hopfner, K. P. & Hornung, V. Molecular mechanisms and cellular functions of cGAS–STING signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521 (2020).
McCully, K. S. Environmental pollution, oxidative stress and thioretinaco ozonide: effects of glyphosate, fluoride and electromagnetic fields on mitochondrial dysfunction in carcinogenesis, atherogenesis and aging. Ann. Clin. Lab. Sci. 50, 408–411 (2020).
Xu, Y., Lindh, C. H., Jönsson, B. A. G., Broberg, K. & Albin, M. Occupational exposure to asphalt mixture during road paving is related to increased mitochondria DNA copy number: a cross-sectional study. Environ. Health 17, 29 (2018).
Rehwinkel, J. & Gack, M. U. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat. Rev. Immunol. 20, 537–551 (2020).
Suen, H. C., Catlin, E. A., Ryan, D. P., Wain, J. C. & Donahoe, P. K. Biochemical immaturity of lungs in congenital diaphragmatic hernia. J. Pediatr. Surg. 28, 471–475 (1993).
Bryant, A. J. et al. The Third Man: DNA sensing as espionage in pulmonary vascular health and disease. Pulm. Circ. 11, 2045894021996574 (2021).
Frémond, M. L. et al. Overview of STING-associated vasculopathy with onset in infancy (SAVI) among 21 patients. J. Allergy Clin. Immunol. Pract. 9, 803–818.e11 (2021).
Motwani, M. et al. Hierarchy of clinical manifestations in SAVI N153S and V154M mouse models. Proc. Natl Acad. Sci. USA 116, 7941–7950 (2019).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Dylong, F. et al. Overactivated epithelial NF-κB disrupts lung development in human and nitrofen CDH. bioRxiv 2023.04.07.536002 https://doi.org/10.1101/2023.04.07.536002 (2023).
Nogueira-Silva, C., Moura, R. S., Esteves, N., Gonzaga, S. & Correia-Pinto, J. Intrinsic catch-up growth of hypoplastic fetal lungs is mediated by interleukin-6. Pediatr. Pulmonol. 43, 680–689 (2008).
Hofmann, A. D., Takahashi, T., Duess, J., Gosemann, J.-H. & Puri, P. Increased expression of activated pSTAT3 and PIM-1 in the pulmonary vasculature of experimental congenital diaphragmatic hernia. J. Pediatr. Surg. 50, 908–911 (2015).
Vasiyani, H. et al. DNA damage induces STING mediated IL-6-STAT3 survival pathway in triple-negative breast cancer cells and decreased survival of breast cancer patients. Apoptosis 27, 961–978 (2022).
Harada, K. et al. Innate immune response to double-stranded RNA in biliary epithelial cells is associated with the pathogenesis of biliary atresia. Hepatology 46, 1146–1154 (2007).
Kutasy, B., Pes, L., Friedmacher, F., Paradisi, F. & Puri, P. Nitrofen increases total retinol levels in placenta during lung morphogenesis in the nitrofen model of congenital diaphragmatic hernia. Pediatr. Surg. Int. 30, 1017–1022 (2014).
Patel, N., Moenkemeyer, F., Germano, S. & Cheung, M. M. H. Plasma vascular endothelial growth factor A and placental growth factor: Novel biomarkers of pulmonary hypertension in congenital diaphragmatic hernia. Am. J. Physiol. - Lung Cell. Mol. Physiol. 308, L378–L383 (2015).
Kell, A. M. & Gale, M. RIG-I in RNA virus recognition. Virology 479–480, 110–121 (2015).
Funding
MM was the holder of a scholarship (MA 8982/1-1) from the “Deutsche Forschungsgemeinschaft e.V.” (DFG - German Research Foundation) during his work on this study; RK is supported by project grants (148797, 178347 and 178387) from the Canadian Institutes of Health Research and is the inaugural Thorlakson Chair in Surgical Research for the University of Manitoba. MJ is a holder of a scholarship (JA 3507/1-1) from the “Deutsche Forschungsgemeinschaft e.V.”.
Author information
Authors and Affiliations
Contributions
MM, WHT, RW and RK: substantial contributions to conception and design. MM, WHT, NDL, SKZ, JA, SH, DP, AO, MJ, RK: acquisition of data, or analysis and interpretation of data. MM, WHT, NDL, AO, MJ, RW, ML, RK: drafting the article or revising it critically for important intellectual content. All authors: Final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Markel, M., Tse, W.H., De Leon, N. et al. Experimental congenital diaphragmatic hernia features an alteration of DNA sensing targets cGAS and STING. Pediatr Res 96, 1666–1672 (2024). https://doi.org/10.1038/s41390-024-03277-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03277-2