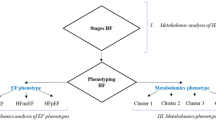
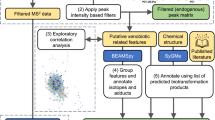
Abstract
Background
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart disease (CCHD) with multifactorial etiology. We aimed to investigate the metabolic profiles of CCHD and their independent contributions to TOF.
Methods
A cohort comprising 42 individuals with TOF and atrial septal defect (ASD) was enrolled. Targeted ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) was employed to systematically analyze metabolite levels and identify TOF-associated metabolic profiles.
Results
Of 370 identified metabolites in tissue and 284 in plasma, over one-third of metabolites showed an association with microbiome. Differential metabolic pathways including amino acids biosynthesis, ABC (ATP-binding cassette) transporters, carbon metabolism, and fatty acid biosynthesis, shed light on TOF biological phenotypes. Additionally, ROC curves identified potential biomarkers, such as erythronic acid with an AUC of 0.868 in plasma, and 3-β-hydroxy-bisnor-5-cholenic acid, isocitric acid, glutaric acid, ortho-Hydroxyphenylacetic acid, picolinic acid with AUC close to 1 in tissue, whereas the discriminative performance of those substances significantly improved when combined with clinical phenotypes.
Conclusions
Distinct metabolic profiles exhibited robust discriminatory capabilities, effectively distinguishing TOF from ASD patients. These metabolites may serve as biomarkers or key molecular players in the intricate metabolic pathways involved in CCHD development.
Impact
-
Distinct metabolic profiles exhibited robust discriminatory capabilities, effectively distinguishing Tetralogy of Fallot from atrial septal defect patients.
-
Similar profiling but inconsistent differential pathways between plasma and tissue.
-
More than one-third metabolites in plasma and tissue are associated with the microbiome.
-
The discovery of biomarkers is instrumental in facilitating early detection and diagnosis of Tetralogy of Fallot.
-
Disturbed metabolism offers insights into interpretation of pathogenesis of Tetralogy of Fallot.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Data will be made available on request.
References
Villafañe, J. et al. Hot topics in tetralogy of Fallot. J. Am. Coll. Cardiol. 62, 2155–2166 (2013).
Shinebourne, E. A., Babu-Narayan, S. V. & Carvalho, J. S. Tetralogy of Fallot: From fetus to adult. Heart (Br. Card. Soc.) 92, 1353–1359 (2006).
Apitz, C., Webb, G. D. & Redington, A. N. Tetralogy of Fallot. Lancet (Lond., Engl.) 374, 1462–1471 (2009).
Starr, J. P. Tetralogy of Fallot: Yesterday and today. World J. Surg. 34, 658–668 (2010).
Michielon, G. et al. Genetic Syndromes and outcome after surgical correction of tetralogy of Fallot. Ann. Thorac. Surg. 81, 968–975 (2006).
Althali, N. J. & Hentges, K. E. Genetic Insights into non-syndromic tetralogy of Fallot. Front. Physiol. 13, 1012665 (2022).
Page, D. J. et al. Whole exome sequencing reveals the major genetic contributors to nonsyndromic tetralogy of Fallot. Circ. Res. 124, 553–563 (2019).
Xu, Z. et al. Efficient plasma metabolic fingerprinting as a novel tool for diagnosis and prognosis of gastric cancer: a large-scale, multicentre study. Gut. 72, 2051-2067 (2023).
Sun, K. et al. Plasma metabolic signatures for Intracranial Aneurysm and its rupture identified by pseudotargeted metabolomics. Clin. Chim. Acta; Int. J. Clin. Chem. 538, 36–45 (2023).
Chen, B. Y. et al. Integrated Omic analysis of human plasma metabolites and microbiota in a hypertension cohort. Nutrients. 15, 2074 (2023).
Bai, Y. et al. Metabolomic interplay between gut microbiome and plasma metabolome in cardiac surgery-associated acute kidney injury. Rapid Commun. mass Spectrom. : RCM 37, e9504 (2023).
Liu, Y. et al. Suppression of Myocardial Hypoxia-inducible Factor-1α compromises metabolic adaptation and impairs cardiac function in patients with cyanotic congenital heart disease during puberty. Circulation 143, 2254–2272 (2021).
Wang, W. et al. Plasma metabolic profiling of patients with tetralogy of fallot. Clin. Chim. acta; Int. J. Clin. Chem. 548, 117522 (2023).
Yu, M. et al. Discovery and validation of potential serum biomarkers for pediatric patients with congenital heart diseases by metabolomics. J. Proteome Res. 17, 3517–3525 (2018).
Hsu, P. C., Maity, S., Patel, J., Lupo, P. J. & Nembhard, W. N. Metabolomics signatures and subsequent maternal health among mothers with a congenital heart defect-affected pregnancy. Metabolites 12 (2022).
Cedars, A. et al. Metabolomic profiling of adults with congenital heart disease. Metabolites 11, 525 (2021).
Pagano, E. et al. Alterations in Metabolites Associated with Hypoxemia in Neonates and Infants with Congenital Heart Disease. Congenit. heart Dis. 15, 251–265 (2020).
Bassareo, P. P. & McMahon, C. J. Metabolomics: A new tool in our understanding of congenital heart disease. Children. 9, 1803 (2022).
Ye, D. et al. Integrative metagenomic and metabolomic analyses reveal gut microbiota-derived multiple hits connected to development of gestational Diabetes Mellitus in humans. Gut microbes 15, 2154552 (2023).
Simić, K. et al. Metabolomic profiling of bipolar disorder by (1)H-Nmr in Serbian patients. Metabolites. 13, 607 (2023).
Goncalves, V. C. et al. Propolis induces cardiac metabolism changes in 6-Hydroxydopamine Animal Model: A dietary intervention as a potential cardioprotective approach in Parkinson’s disease. Front. Pharmacol. 13, 1013703 (2022).
Lin, H. et al. Integrated analysis of the Cecal Microbiome and plasma metabolomics to explore naomaitong and its potential role in changing the intestinal flora and their metabolites in ischemic stroke. Front. Pharmacol. 12, 773722 (2021).
Deidda, M. et al. Metabolomic approach to profile functional and metabolic changes in heart failure. J. Transl. Med. 13, 297 (2015).
Liu, L. et al. Energy metabolism disorder dictates chronic hypoxia damage in heart defect with tetralogy of Fallot. Front. Cardiovasc. Med. 9, 1096664 (2022).
Gall, W. E. et al. Alpha-Hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PloS one 5, e10883 (2010).
Bahado-Singh, R. O. et al. Metabolomic prediction of fetal congenital heart defect in the first trimester. Am. J. Obstet. Gynecol. 211, 240.e241–240.e214 (2014).
Du, D. et al. When N(7)-Methyladenosine modification meets cancer: emerging frontiers and promising therapeutic opportunities. Cancer Lett. 562, 216165 (2023).
Sun, H., Li, K., Liu, C. & Yi, C. Regulation and functions of non-M(6)a mRNA modifications. Nat. Rev. Mol. cell Biol. 24, 714–731 (2023).
Harding, J. J., Hassett, P. C., Rixon, K. C., Bron, A. J. & Harvey, D. J. Sugars including erythronic and threonic acids in human aqueous humour. Curr. Eye Res. 19, 131–136 (1999).
Thomas, I. et al. Integrative analysis of circulating metabolite profiles and magnetic resonance imaging metrics in patients with traumatic brain injury. Int. J. Mol. Sci. 21 (2020).
Jiang, L. et al. Reductive Carboxylation supports Redox Homeostasis during anchorage-independent growth. Nature 532, 255–258 (2016).
Israilova, M. Z. Diagnostic significance of analysis the energy metabolism substrates in biological fluids. Klinicheskaia laboratornaia diagnostika, 22–24 (2002).
Komatsuzaki, S., Ediga, R. D., Okun, J. G., Kölker, S. & Sauer, S. W. Impairment of astrocytic Glutaminolysis in Glutaric Aciduria Type I. J. Inherit. Metab. Dis. 41, 91–99 (2018).
Bosco, M. C. et al. Macrophage activating properties of the Tryptophan Catabolite Picolinic acid. Adv. Exp. Med. Biol. 527, 55–65 (2003).
Melillo, G., Cox, G. W., Radzioch, D. & Varesio, L. Picolinic acid, a catabolite of L-Tryptophan, is a costimulus for the induction of reactive nitrogen intermediate production in murine macrophages. J. Immunol. 150, 4031–4040 (1993).
Santisukwongchote, K., Amornlertwatana, Y., Sastraruji, T. & Jaikang, C. Possible use of blood Tryptophan metabolites as biomarkers for coronary heart disease in sudden unexpected death. Metabolites. 10, 6 (2019).
Majewski, M., Kasica, N., Jakimiuk, A. & Podlasz, P. Toxicity and cardiac effects of acute exposure to tryptophan metabolites on the Kynurenine pathway in early developing Zebrafish (Danio Rerio) embryos. Toxicol. Appl. Pharmacol. 341, 16–29 (2018).
Engelke, U. F. et al. Mitochondrial involvement and erythronic acid as a novel biomarker in transaldolase deficiency. Biochim. et. Biophys. Acta 1802, 1028–1035 (2010).
Liu, X. et al. Disorders of gut microbiota in children with tetralogy of Fallot. Transl. Pediatrics 11, 385–395 (2022).
Agus, A., Clément, K. & Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 70, 1174–1182 (2021).
Witkowski, M., Weeks, T. L. & Hazen, S. L. Gut microbiota and cardiovascular disease. Circ. Res. 127, 553–570 (2020).
Shi, B. et al. Targeting gut microbiota-derived Kynurenine to predict and protect the remodeling of the pressure-overloaded young heart. Sci. Adv. 9, eadg7417 (2023).
Acknowledgements
We are grateful for the support of our colleagues and teachers in the Department of Cardiac Surgery, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences. And we also want to thank all participants in our studies. Fundings This research was supported by the National Key Research and Development Program of China (No. 2022YFC2407406), Guangzhou Science and Technology Planning Project (No. 2023B03J0596), Science and Technology Fundation of Guangzhou Health (No. 2023A031004), 2022 Stability Support for Innovative Capacity Building of Guangdong Provincial Scientific Research Institutions (No. KD022022015).
Author information
Authors and Affiliations
Contributions
Ying Li: Conceptualization; formal analysis; investigation; methodology; visualization, writing-original draft; Miao Tian: Data curation; methodology; visualization; Ziqin Zhou: Conceptualization; data curation; methodology; Jiazichao Tu: Data curation; methodology; Ruyue Zhang: Methodology; formal analysis; Yu Huang: Data curation; Hujun Cui: Data curation; Yong Zhang: Data curation; Jian Zhuang: Writing – review & editing; investigation; Jimei Chen: Conceptualization; writing – review & editing, funding acquisition, project administration; investigation; validation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
According to the guidelines in the Declaration of Helsinki, all patients and their guardians were informed of the study and signed informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Tian, M., Zhou, Z. et al. Integrative metabolomics dictate distinctive signature profiles in patients with Tetralogy of Fallot. Pediatr Res 97, 785–797 (2025). https://doi.org/10.1038/s41390-024-03328-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03328-8