Abstract
Background
With the increase in the number of low birth weight infants, oxygen therapy is more widely used. However, chronic high-concentration oxygen environments lead to hyperoxic lung injury in children, which in turn leads to bronchopulmonary dysplasia (BPD). PGE1 is widely used in the clinic for its ability to inhibit inflammation and improve circulation. Therefore, we further investigated whether PGE-1 has a therapeutic effect on hyperoxic lung injury.
Methods
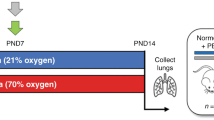
Hyperoxic lung injury model was adopted for investigating the interventional effects and underlying mechanisms of intraperitoneal injection of prostaglandin E1 (PGE-1) on hyperoxic lung injury in newborn rats via relevant experimental techniques, such as Diff-Quick staining, lung wet dry specific gravity measurements, HE staining, TUNEL staining, ELISA, and the Western blot method.
Results
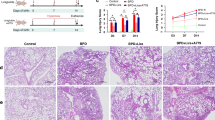
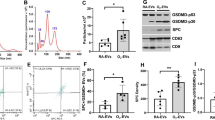
Inflammatory and apoptotic cells in the PGE1-treated group were significantly lower than those in the hyperoxic lung injury group (p < 0.05); and the contents of IL-1β, IL-6 and TNF-α in the treated group were significantly lower than those in the model group (p < 0.05). Caspase-3, CHOP, GRP78 and Bcl-2/Bax protein expression in the treatment group was significantly lower than that in the model group (p < 0.05).
Conclusion
PGE-1 has a therapeutic effect on hyperoxic lung injury in neonatal rats.
Impact
-
PGE1 treatment reduces levels of inflammatory cells and pro-inflammatory cytokines and decreases apoptosis.
-
PGE1 has a therapeutic effect on BPD through the endoplasmic reticulum stress pathway.
-
This study offers the possibility of PGE1 for the treatment of BPD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout















Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Geng, N. et al. Prostaglandin E1 administration for prevention of contrast-induced acute kidney injury: a systematic review and meta-analysis of randomized controlled trials. Medicine 97, e11416 (2018).
Wang, S. & Kaufman, R. J. The impact of the unfolded protein response on human disease. J. Cell Biol. 197, 857–867 (2012).
Tamaki, T. et al. A novel transmembrane protein defines the endoplasmic reticulum stress-induced cell death pathway. Biochem. Biophys. Res. Commun. 486, 149–155 (2017).
Rozpedek, W. et al. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 16, 533–544 (2016).
Fernandes-Alnemri, T., Litwack, G. & Alnemri, E. S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J. Biol. Chem. 269, 30761–30764 (1994).
Rao, R. V. et al. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 514, 122–128 (2002).
Yuan, J., Shaham, S., Ledoux, S., Ellis, H. M. & Horvitz, H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell 75, 641–652 (1993).
Schrör, K. & Hohlfeld, T. Mechanisms of anti-ischemic action of prostaglandin E1 in peripheral arterial occlusive disease. Vasa 33, 119–124 (2004).
Liu, B. et al. Lipo‑prostaglandin E1 modifies cognitive impairment in rats with vascular cognitive impairment by promoting angiogenesis via the VEGF/VEGFR pathway. Mol. Med. Rep. 16, 3117–3124 (2017).
Hafez, T. et al. The effect of intraportal prostaglandin E1 on adhesion molecule expression, inflammatory modulator function, and histology in canine hepatic ischemia/reperfusion injury. J. Surg. Res. 138, 88–99 (2007).
Warner, B. B., Stuart, L. A., Papes, R. A. & Wispé, J. R. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am. J. Physiol. 275, L110–L117 (1998).
Fiebich, B. L. et al. Prostaglandin E2 induces interleukin-6 synthesis in human astrocytoma cells. J. Neurochem. 68, 704–709 (1997).
Yang, M., Chen, Y., Huang, X., Shen, F. & Meng, Y. ETS1 ameliorates hyperoxia-induced bronchopulmonary dysplasia in mice by activating Nrf2/HO-1 mediated ferroptosis. Lung 201, 425–441 (2023).
Xu, C., Xue, D. & Jin, Z. Effects of insulin-like growth factor 1 on hyperoxic lung injury in neonatal rats and its mechanism discussion. Shandong Med 56, 37–39 (2016).
Ozdemir, R. et al. Colchicine protects against hyperoxic lung injury in neonatal rats. Neonatology 102, 265–269 (2012).
Jia, D. et al. Ferroptosis is involved in hyperoxic lung injury in neonatal rats. J. Inflamm. Res. 14, 5393–5401 (2021).
Pritchard, K. A. Jr et al. Role of endoplasmic reticulum stress in impaired neonatal lung growth and bronchopulmonary dysplasia. PLoS ONE 17, e0269564 (2022).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Jin, R. et al. The interaction of S100A16 and GRP78 actives endoplasmic reticulum stress-mediated through the IRE1α/XBP1 pathway in renal tubulointerstitial fibrosis. Cell Death Dis. 12, 942 (2021).
Oyadomari, S. & Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 11, 381–389 (2004).
Lu, M. et al. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 345, 98–101 (2014).
Shakeri, R., Kheirollahi, A. & Davoodi, J. Apaf-1: regulation and function in cell death. Biochimie 135, 111–125 (2017).
Sun, D. K., Wang, L. & Zhang, P. Antitumor effects of chrysanthemin in PC-3 human prostate cancer cells are mediated via apoptosis induction, caspase signalling pathway and loss of mitochondrial membrane potential. Afr. J. Tradit. Complement. Altern. Med. 14, 54–61 (2017).
Ahmed, F. F., Abd El-Hafeez, A. A., Abbas, S. H., Abdelhamid, D. & Abdel-Aziz, M. New 1,2,4-triazole-chalcone hybrids induce caspase-3 dependent apoptosis in A549 human lung adenocarcinoma cells. Eur. J. Med. Chem. 151, 705–722 (2018).
Rogers, C. et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 10, 1689 (2019).
Funding
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
Zhenlin Yang contributed to the performed the experiments and wrote the original draft of the manuscript. Zhengyong Jin and Jinzi Li performed the relevant literature research and revised the manuscript. Jianing Song and Fan Gao contributed to the literature search and processing of the findings. Jingjing Guo, Jiarui Li and Weiwei Zheng contributed reagents, materials, and analysis tools; all authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments were carried out according to the ethical policies and the procedures approved by the Institutional Animal Use and Care Committee of Yanbian University of Science and Technology.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Z., Song, J., Guo, J. et al. Effects of PGE1 on the ERS pathway in neonatal rats with hyperoxic lung injury. Pediatr Res 97, 835–842 (2025). https://doi.org/10.1038/s41390-024-03381-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03381-3
This article is cited by
-
Transcriptomics reveals the underlying mechanism of Prostaglandin E1 in improving severe pneumonia
BMC Pulmonary Medicine (2025)