Abstract
Background
Prechtl’s general movements assessment (GMA) allows visual recognition of movement patterns that, when abnormal (cramped synchronized, or CS), have very high sensitivity in predicting later neuromotor disorders; however, training requirements and subjective perceptions from some clinicians may hinder universal adoption of the GMA in the newborn period.
Methods
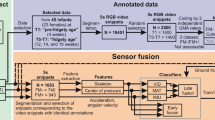
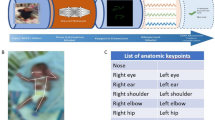
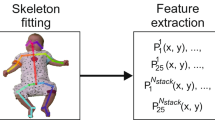
To address this, we used a three-phased approach to design a preliminary and clinically-oriented approach to automated CS GMA detection. 335 hospitalized infants were dually recorded on video and a pressure-sensor mat that collected time, spatial, and pressure data. Video recordings were scored by advanced GMA readers. We then conducted a series of unsupervised machine learning and supervised classification modeling with features extracted from clinician- and mat-driven datasets. Finally, the resulting algorithm was converted to a software interface.
Results
A classification model combining normalization, clustering, and decision tree modeling resulted in the highest sensitivity for CS movements (100%). Results were delivered via the software interface within 20 min of data recording.
Conclusion
The combination of clinical research, machine learning, and repurposing of existing sensor mat technology produced a feasible preliminary approach to automatically detect abnormal GMA in infants while still in the NICU. Further refinements of software and algorithms are needed.
Impact statement
-
Machine learning can differentiate cramped synchronized general movement patterns in the neonatal intensive care unit with good sensitivity and specificity.
-
Increasing access to the GMA through automated detection methods may allow for earlier identification of a greater number of children at high risk for movement delay.
-
Large studies leveraging new artificial intelligence approaches could increase the impact of such detection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data are available upon reasonable request to the corresponding author.
References
Gotardo, J. W. et al. Impact of peri-intraventricular haemorrhage and periventricular leukomalacia in the neurodevelopment of preterms: a systematic review and meta-analysis. PLoS ONE 14, e0223427 (2019).
Hortensius, L. M. et al. Neurodevelopmental consequences of preterm isolated cerebellar hemorrhage: a systematic review. Pediatrics 142, e20180609 (2018).
Milner, K. M., Neal, E. F., Roberts, G., Steer, A. C. & Duke, T. Long-term neurodevelopmental outcome in high-risk newborns in resource-limited settings: a systematic review of the literature. Paediatr. Int. Child Health 35, 227–42, (2015).
Pascal, A. et al. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev. Med. Child Neurol. 60, 342–55, (2018).
Wagenaar, N. et al. Neurodevelopment after perinatal arterial ischemic stroke. Pediatrics 142, e20174164 (2018).
Novak, I. et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. 171, 897–907 (2017).
Peyton, C. & Einspieler, C. General movements: a behavioral biomarker of later motor and cognitive dysfunction in NICU graduates. Pediatr. Ann. 47, e159–e164 (2018).
Kwong, A. K. L., Fitzgerald, T. L., Doyle, L. W., Cheong, J. L. Y. & Spittle, A. J. Predictive validity of spontaneous early infant movement for later cerebral palsy: a systematic review. Dev. Med. Child Neurol. 60, 480–9, (2018).
Ferrari, F. et al. Cramped synchronized general movements in preterm infants as an early marker for cerebral palsy. Arch. Pediatr. Adolesc. Med. 156, 460–7, (2002).
Kodric, J., Sustersic, B. & Paro-Panjan, D. Assessment of general movements and 2.5 year developmental outcomes: pilot results in a diverse preterm group. Eur. J. Paediatr. Neurol. 14, 131–7 (2010).
Ferrari, F., Einspieler, C., Prechtl, H., Bos, A. & Cioni, G. Prechtl’s Method on the Qualitative Assessment of General Movements in Preterm, Term and Young Infants (Mac Keith Press, 2004).
Einspieler, C. & Prechtl, H. F. Prechtl’s assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment. Retard. Dev. Disabil. Res. Rev. 11, 61–7, (2005).
Stoen, R. et al. The predictive accuracy of the general movement assessment for cerebral palsy: a prospective, observational study of high-risk infants in a clinical follow-up setting. J. Clin. Med. 8, 1790 (2019).
Valentin, T., Uhl, K. & Einspieler, C. The effectiveness of training in Prechtl’s method on the qualitative assessment of general movements. Early Hum. Dev. 81, 623–7 (2005).
Guzzetta, A. et al. Does the assessment of general movements without video observation reliably predict neurological outcome? Eur. J. Paediatr. Neurol. 11, 362–7 (2007).
Silva, N. et al. The future of General Movement Assessment: the role of computer vision and machine learning—a scoping review. Res. Dev. Disabil. 110, 103854 (2021).
Maitre, N. et al. General movement assessments in the neonatal intensive care unit improves targeted neuroimaging and follow-up of infants at high-risk for movement disorders. Dev. Med. Child Neurol. 59, 63–63 (2017).
Pascual-Marqui, R. D., Michel, C. M. & Lehmann, D. Segmentation of brain electrical activity into microstates: model estimation and validation. IEEE Trans. Inf. Theory 42, 658–65, (1995).
Murray, M. M., Brunet, D. & Michel, C. M. Topographic ERP analyses: a step-by-step tutorial review. Brain Topogr. 20, 249–64 (2008).
Brunet, D., Murray, M. M. & Michel, C. M. Spatiotemporal analysis of multichannel EEG: CARTOOL. Comput. Intell. Neurosci. 2011, 813870 (2011).
Harris, P. A. et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inf. 95, 103208 (2019).
Byrne, R., Noritz, G., Maitre, N. L. & Group NCHED. Implementation of early diagnosis and intervention guidelines for cerebral palsy in a high-risk infant follow-up clinic. Pediatr. Neurol. 76, 66–71 (2017).
Maitre, N. L. et al. Network implementation of guideline for early detection decreases age at cerebral palsy diagnosis. Pediatrics 145, e20192126 (2020).
McKinney, W. Data structures for statistical computing in Python. In: Proceedings of the 9th Python in Science Conference. 56–61 (2010).
The Pandas Development Team. pandas-dev/pandas: Pandas 1.1.3 (Version 1.1.3): Zenodo; 2020 [1.1.3: https://doi.org/10.5281/zenodo.4067057].
Van Rossum, G., Drake, F. L. Python 3 Reference Manual (CreateSpace, 2009).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–72, (2020).
Harris et al. Array programming with NumPy. Nature 585, 357–362 (2020).
Stoen, R. et al. Computer-based video analysis identifies infants with absence of fidgety movements. Pediatr. Res. 82, 665–70, (2017).
Dey, N., & Ashour, A. Classification and Clustering in Biomedical Signal Processing. Vol xxxvii, p 463 (IGI Global, 2016).
Hubert, L. & Arabie, P. Comparing partitions. J. Classif. 2, 193–218 (1985).
Lloyd, S. Least squares quantization in PCM. IEEE Trans. Inf. Theory 28, 129–37, (1982).
Buitinck, L. et al. (eds) API design for machine learning software: experiences from the scikit-learn project. In Proceedings of the ECML PKDD Workshop: Languages for Data Mining and Machine Learning (2013).
Pedregosa, F. et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn Res. 12, 2825–30, (2011).
Breiman, L., Friedman, J., Olshen, R. A. & Stone, C. J. Classification and regression trees. 358 (Wadsworth & Brooks/Cole Advanced Books & Software, 1984).
Goldberger, J. et al. Neighbourhood components analysis. In: (eds Saul L. K., Weiss Y., Bottou L.) Advances in Neural Information Processing Systems. Vol. 17. 513–520 (MIT Press, 2005).
Wolpert, D. H. Stacked generalization. Neural Netw. 5, 241–59, (1992).
Schmidt, M., Le Roux, N. & Bach, F. Minimizing finite sums with the stochastic average gradient. Math. Program 162, 83–112 (2016).
Bishop, C. M. Pattern Recognition and Machine Learning (Springer, 2006).
Raghuram, K. et al. Automated movement recognition to predict motor impairment in high‐risk infants: a systematic review of diagnostic test accuracy and meta‐analysis. Dev. Med. Child Neurol. 63, 637–48, (2021).
Gravem, D. et al. Assessment of infant movement with a compact wireless accelerometer system. J. Med. Device 6, 021013 (2012).
Adde, L., Helbostad, J. L., Jensenius, A. R., Taraldsen, G. & Stoen, R. Using computer-based video analysis in the study of fidgety movements. Early Hum. Dev. 85, 541–7, (2009).
Kulvicius, T. et al. Infant movement classification through pressure distribution analysis. Commun. Med. 3, 112 (2023).
Philippi, H. et al. Computer-based analysis of general movements reveals stereotypies predicting cerebral palsy. Dev. Med. Child Neurol. 56, 960–7, (2014).
Rahmati, H. et al. Frequency analysis and feature reduction method for prediction of cerebral palsy in young infants. IEEE Trans. Neural Syst. Rehabil. Eng. 24, 1225–34, (2016).
Karch, D. et al. Kinematic assessment of stereotypy in spontaneous movements in infants. Gait Posture 36, 307–311 (2012).
Heinze, F. et al. Movement analysis by accelerometry of newborns and infants for the early detection of movement disorders due to infantile cerebral palsy. Med. Biol. Eng. Comput. 48, 765–72, (2010).
Meinecke, L. et al. Movement analysis in the early detection of newborns at risk for developing spasticity due to infantile cerebral palsy. Hum. Mov. Sci. 25, 125–44, (2006).
Adde, L. et al. Early prediction of cerebral palsy by computer-based video analysis of general movements: a feasibility study. Dev. Med. Child Neurol. 52, 773–778 (2010).
Adde, L., Helbostad, J., Jensenius, A. R., Langaas, M. & Stoen, R. Identification of fidgety movements and prediction of CP by the use of computer-based video analysis is more accurate when based on two video recordings. Physiother. Theory Pract. 29, 469–75, (2013).
Stahl, A. et al. An optical flow-based method to predict infantile cerebral palsy. IEEE Trans. Neural Syst. Rehabil. Eng. 20, 605–14, (2012).
Singh, M. & Patterson, D. J. Involuntary gesture recognition for predicting cerebral palsy in high-risk infants. In: Proceedings of the International Symposium on Wearable Computers (ISWC) Seoul, Korea. 1–8 (IEEE, 2010).
Fan, M., Gravem, D., Cooper, D. & Patterson, D. Augmenting gesture recognition with erlang-cox models to identify neurological disorders in premature babies. In: Proceedings of the ACM Conference on Ubiquitous Computing. 411–420 (Pittsburgh, Pennsylvania, ACM, 2012).
Gao, Y. et al. Towards reliable, automated general movement assessment for perinatal stroke screening in infants using wearable accelerometers. In: Proceedings of the ACM Interact Mob Wearable Ubiquitous Technology (ACM, 2019).
Redd, C. B., Karunanithi, M., Boyd, R. N. & Barber, L. A. Technology-assisted quantification of movement to predict infants at high risk of motor disability: a systematic review. Res. Dev. Disabil. 118, 104071 (2021).
de Vries, N. K. & Bos, A. F. The quality of general movements in the first ten days of life in preterm infants. Early Hum. Dev. 86, 225–9, (2010).
Einspieler, C. et al. The general movement optimality score: a detailed assessment of general movements during preterm and term age. Dev. Med. Child Neurol. 58, 361–8 (2016).
Nakajima, Y., Einspieler, C., Marschik, P. B., Bos, A. F. & Prechtl, H. F. Does a detailed assessment of poor repertoire general movements help to identify those infants who will develop normally? Early Hum. Dev. 82, 53–9 (2006).
Chirigos, A. et al. Prechtl’s General Movements Assessment at writhing age guides MRI use in clinical implementation network. Pediatr. Res. 95, 1188–1190 (2023).
Elliott, C. et al. Early Moves: a protocol for a population-based prospective cohort study to establish general movements as an early biomarker of cognitive impairment in infants. BMJ Open 11. https://doi.org/10.1136/bmjopen-2020-041695 (2021).
Alonzo, C. J. et al. High prevalence of abnormal general movements in hospitalized very low birth weight infants. Am. J. Perinatol. 29, 1541–1547 (2022).
Acknowledgements
We would like to thank Drs. Melissa Murphy, Larken Marra, Mary Ann Nelin, and Dennis Lewandowski for their help in data collection and manuscript preparation.
Funding
This work was supported by a grant from the American Academy for Cerebral Palsy and Developmental Medicine (AACPDM 82131216-Pedal-With-Pete).
Author information
Authors and Affiliations
Contributions
N.L.M. initiated, obtained funding, designed the research, and supervised data collection. C.P.K. collected data. N.L.M. and A.J. developed the algorithms and software tools necessary for the analysis. N.L.M., C.P.K., A.F.D., A.G., and A.J. interpreted the data and wrote the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
A patent has been filed with regard to the detection processes and algorithms.
Informed consent
Informed consent was obtained by the parents of all participants prior to study participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maitre, N.L., Kjeldsen, C.P., Duncan, A.F. et al. Automated detection of abnormal general movements from pressure and positional information in hospitalized infants. Pediatr Res 97, 598–607 (2025). https://doi.org/10.1038/s41390-024-03387-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03387-x