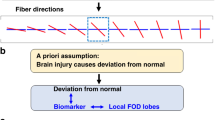
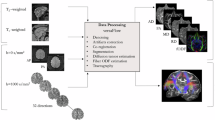
Abstract
Diffusion MRI (dMRI) enables studying the complex architectural organization of the brain’s white matter (WM) through virtual reconstruction of WM fiber tracts (tractography). Despite the anticipated clinical importance of applying tractography to study structural connectivity and tract development during the critical period of rapid infant brain maturation, detailed descriptions on how to approach tractography in young infants are limited. Over the past two decades, tractography from infant dMRI has mainly been applied in research settings and focused on diffusion tensor imaging (DTI). Only few studies used techniques superior to DTI in terms of disentangling information on the brain’s organizational complexity, including crossing fibers. While more advanced techniques may enhance our understanding of the intricate processes of normal and abnormal brain development and extensive knowledge has been gained from application on adult scans, their applicability in infants has remained underexplored. This may partially be due to the higher technical requirements versus the need to limit scan time in young infants. We review various previously described methodological practices for tractography in the infant brain (0–2 years-of-age) and provide recommendations to optimize advanced tractography approaches to enable more accurate reconstructions of the brain WM’s complexity.
Impact
-
Diffusion tensor imaging is the technique most frequently used for fiber tracking in the developing infant brain but is limited in capability to disentangle the complex white matter organization.
-
Advanced tractography techniques allow for reconstruction of crossing fiber bundles to better reflect the brain’s complex organization. Yet, they pose practical and technical challenges in the fast developing young infant’s brain. Methods on how to approach advanced tractography in the young infant’s brain have hardly been described.
-
Based on a literature review, recommendations are provided to optimize tractography for the developing infant brain, aiming to advance early diagnosis and neuroprotective strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information Files.
References
Hüppi, P. S. & Dubois, J. Diffusion tensor imaging of brain development. Semin. Fetal Neonatal Med. 11, 489–497 (2006).
Emsell, L., Van Hecke, W. & Tournier, J. Introduction to diffusion tensor imaging. In: Diffusion Tensor Imaging (eds Van Hecke, W. et al.) 7–19 (Springer, 2016).
O’Donnell, L. J. & Westin, C. An introduction to diffusion tensor image analysis. Neurosurg. Clin. N. Am. 22, 185–196 (2011).
Van Hecke, W. & Emsell, L. Strategies and challenges in DTI analysis. In Diffusion Tensor Imaging (eds Van Hecke, W. et al.) 153–173 (Springer, 2016).
Kurtcan, S. et al. Diffusion tensor imaging parameters in children with acute hyperammonemic encephalopathy due to urea cycle enzyme defects and organic acidemia. Curr. Med. Imaging Rev. 14, 837–844 (2018).
Froeling, M., Pullens, P. & Leemans, A. DTI analysis methods: Region of interest analysis. In: Diffusion Tensor Imaging (eds Van Hecke, W. et al.) 175–182 (Springer, 2016).
Dhollander, T. et al. Fixel-based analysis of diffusion MRI: Methods, applications, challenges and opportunities. NeuroImage 241, 118417 (2021).
Van Hecke, W., Leemans, A. & Emsell, L. DTI analysis methods: Voxel-based analysis. In: Diffusion Tensor Imaging (eds Van Hecke, W. et al.) 183–203 (Springer, 2016).
Caan, M. W. A. DTI analysis methods: Fibre tracking and connectivity. In Diffusion Tensor Imaging (eds Van Hecke W. et al.) 205–228 (Springer, 2016).
Massaro, A. N. et al. White matter tract integrity and developmental outcome in newborn infants with hypoxic-ischemic encephalopathy treated with hypothermia. Dev. Med. Child. Neurol. 57, 441–448 (2015).
van Pul, C., Buijs, J., Vilanova, A., Roos, F. G. & Wijn, P. F. Infants with perinatal hypoxic ischemia: Feasibility of fiber tracking at birth and 3 months. Radiology 240, 203–214 (2006).
Jaimes, C. et al. Probabilistic tractography-based thalamic parcellation in healthy newborns and newborns with congenital heart disease. J. Magn. Reson. Imaging 47, 1626–1637 (2017).
Langer, N. et al. White matter alterations in infants at risk for developmental dyslexia. Cereb. Cortex. 27, 1027–1036 (2015).
Liu, J. et al. Altered lateralization of dorsal language tracts in 6‐week‐old infants at risk for autism. Dev. Sci. 22, e12768 (2019).
Chang, S., Garnett, E. O., Etchell, A. & Chow, H. M. Functional and neuroanatomical bases of developmental stuttering: Current insights. Neuroscientist 25, https://doi.org/10.1177/1073858418803594 (2018).
Feldman, H. M., Yeatman, J. D., Lee, E. S., Barde, L. H. F. & Gaman-Bean, S. Diffusion tensor imaging: A review for pediatric researchers and clinicians. J. Dev. Behav. Pediatr. 31, 346–356 (2010).
Yendiki, A. et al. Post mortem mapping of connectional anatomy for the validation of diffusion MRI. NeuroImage 256, 119146 (2022).
Farquharson, S., Tournier, J. High angular resolution diffusion imaging. In: Diffusion Tensor Imaging (eds Van Hecke W. et al.) 383–418 (Springer, 2016).
Basser, P. J. & Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. Imaging 111, 209–2019 (1996).
Yang, J. et al. Reliable dual tensor model estimation in single and crossing fibers based on jeffreys prior. PloS One 11 https://doi.org/10.1371/journal.pone.0164336 (2016).
Veraart, J., Sijbers, J. Diffusion kurtosis imaging. In Diffusion Tensor Imaging (eds Van Hecke W. et al.) 407–440 (Springer, 2016).
Lazar, M., Jensen, J. H., Xuan, L. & Helpern, J. A. Estimation of the orientation distribution function from diffusional kurtosis imaging. Magn. Reson. Med. 60, 774–781 (2008).
Behrens, T. E. J. et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 50, 1077–1088 (2003).
Behrens, T. E. J., Berg, H. J., Jbabdi, S., Rushworth, M. F. S. & Woolrich, M. W. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage 34, 144–155 (2007).
Tuch, D. S. Q-ball imaging. Magn. Reson. Med. 52, 1358–1372 (2004).
Zhang, H., Schneider, T., Wheeler-Kingshott, C. A. & Alexander, D. C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61, 1000–1016 (2012).
Jeurissen, B., Descoteaux, M., Mori, S. & Leemans, A. Diffusion MRI fiber tractography of the brain. NMR Biomed. 32, e3785 (2019).
Jones, D. K. Challenges and limitations of quantifying brain connectivity in vivo with diffusion MRI. Imaging Med. 2, 341–355 (2010).
Sotiropoulos, S. N., Bai, L., Morgan, P. S., Constantinescu, C. S. & Tench, C. R. Brain tractography using Q-ball imaging and graph theory: Improved connectivities through fibre crossings via a model-based approach. NeuroImage 49, 2444–2456 (2010).
Batalle, D., Edwards, A. D. & O’muircheartaigh, J. Annual research review: Not just a small adult brain: Understanding later neurodevelopment through imaging the neonatal brain. J. Child. Psychol. Psychiatr. 59, 350–371 (2017).
Dubois, J. et al. The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience 276, 48–71 (2014).
Partridge, S. C. et al. Diffusion tensor imaging: Serial quantitation of white matter tract maturity in premature newborns. NeuroImage 22, 1302–1314 (2004).
Berman, J. I. et al. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. NeuroImage 27, 862–871 (2005).
Dubois, J., Hertz-Pannier, L., Dehaene-Lambertz, G., Cointepas, Y. & Le Bihan, D. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: A feasibility study using quantitative diffusion tensor imaging and tractography. NeuroImage 30, 1121–1132 (2006).
Kaur, S., Powell, S., He, L., Pierson, C. R. & Parikh, N. A. Reliability and repeatability of quantitative tractography methods for mapping structural white matter connectivity in preterm and term infants at term-equivalent age. PLoS One 9, e85807 (2014).
Zhai, G., Lin, W., Wilber, K. P., Gerig, G. & Gilmore, J. H. Comparisons of regional white matter diffusion in healthy neonates and adults performed with a 3.0-T head-only MR imaging unit. Radiology 229, 673–681 (2003).
Adibpour, P., Lebenberg, J., Kabdebon, C., Dehaene-Lambertz, G. & Dubois, J. Anatomo-functional correlates of auditory development in infancy. Dev. Cogn. Neurosci. 42, 100752 (2020).
Anblagan, D. et al. Tract shape modeling detects changes associated with preterm birth and neuroprotective treatment effects. NeuroImage Clin. 8, 51–58 (2015).
Bastiani, M. et al. Automated processing pipeline for neonatal diffusion MRI in the developing human connectome project. NeuroImage 185, 750–763 (2018).
Akazawa, K. et al. Probabilistic maps of the white matter tracts with known associated functions on the neonatal brain atlas: Application to evaluate longitudinal developmental trajectories in term-born and preterm-born infants. NeuroImage 128, 167–179 (2016).
Fonseca, L. et al. Automatic atlas-based segmentation of brain white matter in neonates at risk for neurodevelopmental disorders. In Modeling, Analysis, and Visualization of Anisotropy. Mathematics and Visualization (eds Schultz T. et al.) 355–372 (Springer, Cham Switzerland, 2017) .
Goodlett, C. B., Fletcher, P. T., Gilmore, J. H. & Gerig, G. Group analysis of DTI fiber tract statistics with application to neurodevelopment. NeuroImage 45, S133–S142 (2009).
Tam, E. W. Y. et al. Hyperglycemia associated with acute brain injury in neonatal encephalopathy. NeuroImage Clin. 32, 102835 (2021).
Quinones, J. F. et al. Fiber tracing and microstructural characterization among audiovisual integration brain regions in neonates compared with young adults. NeuroImage 254, 119141 (2022).
Rajagopal, A. et al. White matter microstructural abnormality in children with hydrocephalus detected by probabilistic diffusion tractography. AJRN. Am. J. Neuroradiol. 34, 2379–2385 (2013).
Eixarch, E., Muñoz-Moreno, E., Bargallo, N., Batalle, D. & Gratacos, E. Motor and cortico-striatal-thalamic connectivity alterations in intrauterine growth restriction. Am. J. Obstet. Gynecol. 214, 725.e1–725.e9 (2016).
Kelly, J. P. et al. Cerebral visual impairment characterized by abnormal visual orienting behavior with preserved visual cortical activation. Invest. Ophthalmol. Vis. Sci. 62, 15 (2021).
Nieuwets, A. et al. Post-hemorrhagic ventricular dilatation affects white matter maturation in extremely preterm infants. Pediatr. Res. 92, 225–232 (2021).
Raguž, M. et al. Structural changes in the cortico-ponto-cerebellar axis at birth are associated with abnormal neurological outcomes in childhood. Clin. Neuroradiol. 31, 1005–1020 (2021).
Wang, S., Fan, G., Xu, K. & Wang, C. Potential of diffusion tensor MR imaging in the assessment of cognitive impairments in children with periventricular leukomalacia born preterm. Eur. J. Radiol. 82, 158–164 (2012).
Jang, Y. H. et al. Abnormal thalamocortical connectivity of preterm infants with elevated thyroid stimulating hormone identified with diffusion tensor imaging. Sci. Rep. 12, 9257 (2022).
Sa de Almeida, J. et al. Music enhances structural maturation of emotional processing neural pathways in very preterm infants. NeuroImage 207, 116391 (2020).
Toselli, B. et al. Improvement of white matter tract reconstruction with constrained spherical deconvolution and track-density mapping in low angular resolution data: A pediatric study and literature review. Front. Pediatr. 5, 182 (2017).
Paydar, A. et al. Diffusional kurtosis imaging of the developing brain. AJNR. Am. J. Neuroradiol. 35, 808–814 (2014).
Tamilia, E. et al. Nutritive sucking abnormalities and brain microstructural abnormalities in infants with established brain injury: A pilot study. J. Perinatol. 39, 1498–1508 (2019).
van Kooij, B. et al. Fiber tracking at term displays gender differences regarding cognitive and motor outcome at 2 years of age in preterm infants. Pediatr. Res. 70, 626–632 (2011).
Lockwood Estrin, G. et al. White and grey matter development in utero assessed using motion-corrected diffusion tensor imaging and its comparison to ex utero measures. MAGMA 32, 473–485 (2019).
Sreedharan, R. M., Aiyappan, S. & Roy, N. Alteration in the number and integrity of white matter tracts in the preterm: A quantitative diffusion tensor imaging and diffusion fibre tractography in children. Indian J. Radiol. Imaging 27, 119–124 (2017).
Pecheva, D. et al. Recent advances in diffusion neuroimaging: Applications in the developing preterm brain. F1000 Res. 7, 1326 (2018).
Parker, G. D. et al. A pitfall in the reconstruction of fibre ODFs using spherical deconvolution of diffusion MRI data. NeuroImage 65, 433–448 (2013).
Ogawa, C. et al. Cytotoxic edema at onset in west syndrome of unknown etiology: A longitudinal diffusion tensor imaging study. Epilepsia 59, 440–448 (2018).
Whitehead, M. T., Raju, A. & Choudhri, A. F. Normal centrolineal myelination of the callosal splenium reflects the development of the cortical origin and size of its commissural fibers. Neuroradiology 56, 333–338 (2014).
Zöllei, L., Jaimes, C., Saliba, E., Grant, P. E. & Yendiki, A. TRActs constrained by UnderLying INfant anatomy (TRACULInA): An automated probabilistic tractography tool with anatomical priors for use in the newborn brain. NeuroImage 199, 1–17 (2019).
Posner, J. et al. Alterations in amygdala–prefrontal circuits in infants exposed to prenatal maternal depression. Transl. Psychiatry 6, 1–8 (2016).
Teli, R. et al. Postnatal microstructural developmental trajectory of corpus callosum subregions and relationship to clinical factors in very preterm infants. Sci. Rep. 7550, 1–12 (2018).
Song, J. W. et al. How accurate are prenatal tractography results? A postnatal in vivo follow-up study using diffusion tensor imaging. Pediatr. Radiol. 48, 486–498 (2018).
de Bruïne, F. T. et al. Tractography of developing white matter of the internal capsule and corpus callosum in very preterm infants. Eur. Radiol. 21, 538–547 (2010).
de Planque, C. A. et al. A diffusion tensor imaging analysis of frontal lobe white matter microstructure in trigonocephaly patients. Pediatr. Neurol. 131, 42–48 (2022).
Lee, S. et al. Diffusion tensor MR imaging visualizes the altered hemispheric fiber connection in callosal dysgenesis. AJNR Am. J. Neuroradiol. 25, 25–28 (2004).
Assaf, Y. & Basser, P. J. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. NeuroImage 27, 48–58 (2005).
Hutter, J. et al. Time-efficient and flexible design of optimized multishell HARDI diffusion. Magn. Reson. Med. 79, 1276–1292 (2018).
Ní Bhroin, M. et al. Reduced structural connectivity in cortico-striatal-thalamic network in neonates with congenital heart disease. NeuroImage Clin. 28, 102423 (2020).
Taoudi-Benchekroun, Y. et al. Predicting age and clinical risk from the neonatal connectome. NeuroImage 257, 119319 (2022).
Sotiropoulos, S. N. et al. Advances in diffusion MRI acquisition and processing in the human connectome project. NeuroImage 80, 125–143 (2013).
Tournier, J., Calamante, F. & Connelly, A. Determination of the appropriate b value and number of gradient directions for high-angular-resolution diffusion-weighted imaging. NMR Biomed. 26, 1775–1786 (2013).
Pannek, K., Guzzetta, A., Colditz, P. B. & Rose, S. E. Diffusion MRI of the neonate brain: Acquisition, processing and analysis techniques. Pediatr. Radiol. 42, 1169–1182 (2012).
Tax, CMW, Vos, SB, Leemans, A. Checking and correcting DTI data. In: Diffusion Tensor Imaging: A Practical Handbook (eds Van Hecke W et al.) 127–150 https://doi.org/10.1007/978-1-4939-3118-7_7 (Springer, 2016).
Tax, C. M. W., Bastiani, M., Veraart, J., Garyfallidis, E. & Okan Irfanoglu, M. What’s new and what’s next in diffusion MRI preprocessing. NeuroImage 249, 118830 (2022).
Shi, Y. & Toga, A. W. Connectome imaging for mapping human brain pathways. Mol. Psychiatry 22, 1230–1240 (2017).
Jeurissen, B., Tournier, J., Dhollander, T., Connelly, A. & Sijbers, J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage 103, 411–426 (2014).
Dell’Acqua, F. et al. A model-based deconvolution approach to solve fiber crossing in diffusion-weighted MR imaging. TBME 54, 462–472 (2007).
Schilling, K. G. et al. Challenges in diffusion MRI tractography – lessons learned from international benchmark competitions. Magn. Reson. Imaging 57, 194–209 (2019).
Calamuneri, A. et al. White matter tissue quantification at low b-values within constrained spherical deconvolution framework. Front. Neurol. 9, 1–14 (2018).
Daducci, A., Dal Palu, A., Lemkaddem, A. & Thiran, J. COMMIT: Convex optimization modeling for microstructure informed tractography. TMI 34, 246–257 (2015).
Shafieizargar, B. et al. ADEPT: Accurate diffusion echo-planar imaging with multi-contrast shoTs. Magn. Reson. Med. 89, 396–410 (2023).
Wasserthal, J., Neher, P. & Maier-Hein, K. H. TractSeg - fast and accurate white matter tract segmentation. NeuroImage 183, 239–253 (2018).
Cai, L. Y. et al. Implementation considerations for deep learning with diffusion MRI streamline tractography. Presented at the Medical Imaging With Deep Learning Conference; 10–12 July 2023.
Jiang, H., van Zijl, P. C. M., Kim, J., Pearlson, G. D. & Mori, S. DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Comput. Methods Prog. Biomed. 81, 106–116 (2006).
Bassi, L. et al. Diffusion tensor imaging in preterm infants with punctate white matter lesions. Pediatr. Res. 69, 561–566 (2011).
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. Fsl. NeuroImage 62, 782 (2011).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23, S208–S219 (2004).
Bell, K. A. et al. Associations of macronutrient intake determined by point-of-care human milk analysis with brain development among very preterm infants. Children 9, 969 (2022).
CRL. Computational radiology kit (CRKIT), http://crl.med.harvard.edu/software/, Accessed on 09 August (2023).
Campbell, K. S. J. et al. Prenatal antidepressant exposure and sex differences in neonatal corpus callosum microstructure. Dev. Psychobiol. 63, e22125 (2021).
Yeh, F., Verstynen, T. D., Wang, Y., Fernández-Miranda, J. C. & Tseng, W. I. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PloS One 8, e80713 (2013).
Ceschin et al. Developmental synergy between thalamic structure and interhemispheric connectivity in the visual system of preterm infants. NeuroImage Clin. 8, 462–472 (2015).
Cha, J. H. et al. Altered microstructure of the splenium of corpus callosum is associated with neurodevelopmental impairment in preterm infants with necrotizing enterocolitis. Ital. J. Pediatr. 48, 1–10 (2022).
de Groot, M. et al. Improving alignment in tract-based spatial statistics: Evaluation and optimization of image registration. NeuroImage Clin. 76, 400–411 (2013).
Dubner, S. E., Rose, J., Bruckert, L., Feldman, H. M. & Travis, K. E. Neonatal white matter tract microstructure and 2-year language outcomes after preterm birth. NeuroImage Clin. 28, 102446 (2020).
Yeatman, J. D., Dougherty, R. F., Myall, N. J., Wandell, B. A. & Feldman, H. M. Tract profiles of white matter properties: Automating fiber-tract quantification. PLoS One 7, e49790 (2012).
Mori, S., Crain, B. J., Chacko, V. P. & Van Zijl, P. C. M. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 45, 265–269 (1999).
ASCLEPIOS. MedINRIA, http://www-sop.inria.fr/asclepios/software/MedINRIA/, Accessed on 09 August (2023).
Gilmore, J. H. et al. Early postnatal development of corpus callosum and corticospinal white matter assessed with quantitative tractography. AJNR. Am. J. Neuroradiol. 28, 1789–1795 (2007).
Fillard, P., Gerig, G. Analysis tool for diffusion tensor MRI. In Proceedings of the Medical Image Computing and Computer Assisted Intervention, 2879 (MICCAI, 2003).
Hasegawa, T. et al. Development of corpus callosum in preterm infants is affected by the prematurity: In vivo assessment of diffusion tensor imaging at term-equivalent age. Pediatr. Res. 69, 249–254 (2011).
Hasegawa, T. et al. Cerebellar peduncle injury predicts motor impairments in preterm infants: A quantitative tractography study at term-equivalent age. Brain Dev. 40, 743–752 (2018).
Huang, H. et al. White and gray matter development in human fetal, newborn and pediatric brains. NeuroImage 33, 27–38 (2006).
Jiang, H. H. et al. Early diagnosis of spastic cerebral palsy in infants with periventricular white matter injury using diffusion tensor imaging. AJNR. Am. J. Neuroradiol. 40, 162–168 (2018).
Karimi, D. & Gholipour, A. Diffusion tensor estimation with transformer neural networks. Artif. Intell. Med. 130, 102330 (2022).
Garyfallidis, E. Towards an accurate brain tractography. PhD thesis (University of Cambridge, 2013).
Wang, E. Diffusion tensor imaging (DTI) tractography. In CyberKnife NeuroRadiosurgery (eds Conti A. et al.) 141–153 (Springer, 2020).
Liu, Y. et al. Structural asymmetries in motor and language networks in a population of healthy preterm neonates at term equivalent age: A diffusion tensor imaging and probabilistic tractography study. NeuroImage 51, 783–788 (2010).
Makki, M. I. & Hagmann, C. Regional differences in interhemispheric structural fibers in healthy, term infants. J. Neurosci. Res. 95, 876–884 (2017).
Mohammad, S. A. & Abdelkhalek, H. S. Nonketotic hyperglycinemia: Spectrum of imaging findings with emphasis on diffusion-weighted imaging. Neuroradiology 59, 1155–1163 (2017).
Morita, T. et al. Low-grade intraventricular hemorrhage disrupts cerebellar white matter in preterm infants: Evidence from diffusion tensor imaging. Neuroradiology 57, 507–514 (2015).
Ng, S. M. et al. Effect of thyroxine on brain microstructure in extremely premature babies: Magnetic resonance imaging findings in the TIPIT study. Pediatr. Radiol. 44, 987–996 (2014).
Leemans A., Jeurissen B., Sijbers J., Jones D. K. ExploreDTI: A graphical toolbox for processing, analyzing, and visualizing diffusion MR data. In Proceedings of the International Society for Magnetic Resonance in Medicine (ISMRM, 2009).
Masutani, Y., Aoki, S., Abe, O., Hayashi, N. & Otomo, K. MR diffusion tensor imaging: Recent advance and new techniques for diffusion tensor visualization. Eur. J. Radiol. 46, 53–66 (2003).
Packman, A. et al. White matter connectivity in neonates at risk of stuttering: Preliminary data. Neurosci. Lett. 781, 136655 (2022).
Yushkevich, P. et al. A tract-specific approach to assessing white matter in preterm infants. NeuroImage 157, 675–694 (2017).
Rha, D., Chang, W. H., Kim, J., Sim, E. G. & Park, E. S. Comparing quantitative tractography metrics of motor and sensory pathways in children with periventricular leukomalacia and different levels of gross motor function. Neuroradiology 54, 615–621 (2011).
Rollins, N. K., Booth, T. N. & Chahrour, M. H. Variability of ponto-cerebellar fibers by diffusion tensor imaging in diverse brain malformations. J. Child Neurol. 32, 271–285 (2017).
Sakaue, S. et al. Low‐grade IVH in preterm infants causes cerebellar damage, motor, and cognitive impairment. Pediatr. Int. 63, 1327–1333 (2021).
Taylor, P. A. et al. A DTI-based tractography study of effects on brain structure associated with prenatal alcohol exposure in newborns. Hum. Brain Mapp. 36, 170–186 (2015).
Taylor, P. A. & Saad, Z. S. FATCAT: (An efficient) functional and tractographic connectivity analysis toolbox. Brain Connect 3, 523–535 (2013).
Thompson, D. K. et al. Accelerated corpus callosum development in prematurity predicts improved outcome. Hum. Brain Mapp. 36, 3733–3748 (2015).
Wang, S., Fan, G. G., Xu, K. & Wang, C. Altered microstructural connectivity of the superior and middle cerebellar peduncles are related to motor dysfunction in children with diffuse periventricular leucomalacia born preterm: A DTI tractography study. Eur. J. Radiol. 83, 997–1004 (2014).
Warton, F. L. et al. Prenatal methamphetamine exposure is associated with corticostriatal white matter changes in neonates. Metab. Brain. Dis. 33, 507–522 (2017).
Weinstein, M. et al. The motor and visual networks in preterm infants: An fMRI and DTI study. Brain Res. 1642, 603–611 (2016).
Yoo, S. et al. In vivo visualization of white matter fiber tracts of preterm- and term-infant brains with diffusion tensor magnetic resonance imaging. Invest. Radiol. 40, 110–115 (2005).
Bravo, E. K., White, M. L., Olney, A. H., McAllister, J. L. & Zhang, Y. D. Novel proximal 14q deletion: Clinical and diffusion tensor imaging tractography findings in a patient with lissencephaly, agenesis of the corpus callosum, and septo-optic dysplasia. AJNR Am. J. Neuroradiol. 33, E16–E18 (2012).
DeGrazia, M. et al. Brain characteristics noted prior to and following cranial orthotic treatment. Child Neurol. Open 7, 1–11 (2020).
Gunbey, H. P., Bilgici, M. C., Aslan, K., Aygün, C. & Celik, H. Ectopic cerebellar tissue of the posterior cranial fossa: Diffusion tensor tractography and MR spectroscopy findings. Childs Nerv. Syst. 32, 195–198 (2015).
Lee, S. & Kim, J. Diffusion tensor imaging of heterotopia: Changes of fractional anisotropy during radial migration of neurons. Yonsei Med. J. 51, 590–593 (2010).
Ormitti, F. et al. Diffusion tensor MR imaging tractography of the pyramidal tracts and corpus callosum in children with right-sided congenital hemiparesis: A case report. Neuroradiol. J. 23, 172–176 (2010).
Rollins, N. Semilobar holoprosencephaly seen with diffusion tensor imaging and fiber tracking. Am. J. Neuroradiol. 26, 2148–2152 (2005).
Rollins, N., Reyes, T. & Chia, J. Diffusion tensor imaging in lissencephaly. AJNR Am. J. Neuroradiol. 26, 1583–1586 (2005).
Ball, G. et al. The influence of preterm birth on the developing thalamocortical connectome. Cortex 49, 1711–1721 (2013).
Ball, G. et al. Thalamocortical connectivity predicts cognition in children born preterm. Cereb. Cortex 25, 4310–4318 (2015).
Sotiropoulos, S. N. et al. Fusion in diffusion MRI for improved fibre orientation estimation: An application to the 3T and 7T data of the human connectome project. NeuroImage 134, 396–409 (2016).
Baxter, L. et al. Functional and diffusion MRI reveal the neurophysiological basis of neonates’ noxious-stimulus evoked brain activity. Nat. Commun. 12, 1–14 (2021).
Braga, R. M. et al. Development of the corticospinal and callosal tracts from extremely premature birth up to 2 years of age. PloS one 10, 1–15 (2015).
Parikh, N. A., Hershey, A. & Altaye, M. Early detection of cerebral palsy using sensorimotor tract biomarkers in very preterm infants. Pediatr. Neurol. 98, 53–60 (2019).
Zubiaurre-Elorza, L. et al. Auditory structural connectivity in preterm and healthy term infants during the first postnatal year. Dev. Psychobiol. 60, 256–264 (2018).
Chandwani, R., Kline, J. E., Harpster, K., Tkach, J. & Parikh, N. A. Early micro‐ and macrostructure of sensorimotor tracts and development of cerebral palsy in high risk infants. Hum. Brain Mapp. 42, 4708–4721 (2021).
Tournier, J. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 202, 116137 (2019).
Grotheer, M. et al. White matter myelination during early infancy is linked to spatial gradients and myelin content at birth. Nat. Commun. 13, 1–12 (2022).
Tournier, J., Calamante, F. & Connelly, A. MRtrix: Diffusion tractography in crossing fiber regions. Int. J. Imaging Syst. Technol. 22, 53–66 (2012).
Kimpton, J. A. et al. Diffusion magnetic resonance imaging assessment of regional white matter maturation in preterm neonates. Neuroradiology 63, 573–583 (2020).
Pieterman, K. et al. Cerebello-cerebral connectivity in the developing brain. Brain. Struct. Funct. 222, 1625–1634 (2017).
Salvan, P. et al. Language ability in preterm children is associated with arcuate fasciculi microstructure at term. Hum. Brain Mapp. 38, 3836–3847 (2017).
Tournier, J., Calamante, F., & Connelly, A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. In Proceedings of the International Society for Magnetic Resonance in Medicine (ISMRM, 2010).
Vaher, K. et al. General factors of white matter microstructure from DTI and NODDI in the developing brain. NeuroImage 254, 119169 (2022).
Li, M. et al. How does microstructural in intra-axonal and extra-axonal space change in preterm infants with punctate white matter lesions? In Proceedings of the International Society for Magnetic Resonance in Medicine (ISMRM, 2020);
Cohen, A. H. et al. Development of human white matter fiber pathways: From newborn to adult ages. Int. J. Dev. Neurosci. 50, 26–38 (2016).
Kulikova, S. et al. Multi-parametric evaluation of the white matter maturation. Brain. Struct. Funct. 220, 3657–3672 (2014).
Duclap, D. et al. Connectomist-2.0: A novel diffusion analysis toolbox for BrainVISA. In Proceedings of the 29th European Society for Magnetic Resonance in Medicine and Biology meeting (ESMRMB, 2012).
Song, J. W. et al. Asymmetry of white matter pathways in developing human brains. Cereb. Cortex 25, 2883–2893 (2015).
Re, T. J. et al. Magnetic resonance fiber tracking in a neonate with hemimegalencephaly. J. Neuroimaging 25, 844–847 (2015).
Smith, S. M. et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage 31, 1487–1505 (2006).
Van Essen, D. C. et al. The WU-minn human connectome project: An overview. NeuroImage 80, 62–79 (2013).
Yeh, F., Wedeen, V. J. & Tseng, W. I. Generalized Q-sampling imaging. IEEE Trans. Med. Imaging 29, 1626–1635 (2010).
Funding
A.S.V. is jointly supported by the Royal Netherlands Academy of Arts and Sciences (KNAW, Amsterdam, The Netherlands) ter Meulen Fund [KNAWWF/1327/TMB202126], Isala Science and Innovation Fund (Zwolle, the Netherlands) and Science Fund Medical Specialist Isala (Zwolle, the Netherlands). L.M.L.’s research program is jointly supported by the Alberta Children’s Hospital Foundation, Alberta Children’s Hospital Research Institute and the Calgary Health Foundation (Calgary, Canada). C.M.W.T. is supported by the Wellcome Trust [215944/Z/19/Z] and a Veni grant (17331) from the Dutch Research Council (NWO). The sponsors had no role in the design and conduct of the study, the collection, management, analysis and interpretation of the data, the preparation, review and approval of the manuscript, or the decision to submit the manuscript for publication. For the purpose of open access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
Anouk Verschuur: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, validation, visualization, writing – original draft, writing – review & editing. Regan King: Conceptualization, data curation, investigation, methodology, validation, writing – original draft, writing – review & editing. Chantal Tax: Conceptualization, methodology, supervision, validation, writing – review & editing. Martijn Boomsma: Conceptualization, funding acquisition, methodology, supervision, validation, writing – review & editing. Gerda Meijler: Conceptualization, funding acquisition, methodology, supervision, validation, writing – review & editing. Alexander Leemans: Conceptualization, methodology, supervision, validation, writing – review & editing. Lara Leijser: Conceptualization, data curation, funding acquisition, investigation, methodology, supervision, validation, writing – original draft, writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verschuur, A.S., King, R., Tax, C.M.W. et al. Methodological considerations on diffusion MRI tractography in infants aged 0–2 years: a scoping review. Pediatr Res 97, 880–897 (2025). https://doi.org/10.1038/s41390-024-03463-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03463-2
This article is cited by
-
Small brains but big challenges: white matter tractography in early life samples
Brain Structure and Function (2025)