Abstract
Background
Intermittent hypoxia (IH) and oxidative stress play key roles in gut dysbiosis and inflammation. We tested the hypothesis that increasing numbers of daily IH episodes cause microbiome dysbiosis and severe gut injury.
Methods
Neonatal rats were exposed to hyperoxia (Hx), growth restriction, and IH. For IH, pups were exposed to 2–12 daily episodes from birth (P0) to postnatal day 7 (7D) or P0-P14 (14D), with or without recovery in room air (RA) until P21. Animals raised in RA from P0 to P21 served as normoxia controls. Stool was expressed from the large intestines for microbiome analysis, and tissue samples were assessed for histopathology and biomarkers of inflammation.
Results
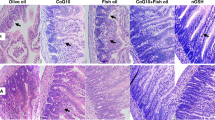
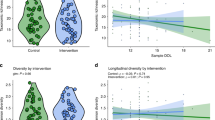
Hx and IH caused a significant reduction in the number and diversity of organisms. The severity of gut injury and levels of inflammatory cytokines and TLR4 increased, while total glutathione (tGSH) declined, with increasing daily IH episodes. The number of organisms correlated with the villi number (p < 0.05) and tGSH depletion (p < 0.001).
Conclusions
The critical number of daily IH episodes that the newborn gut may sustain is 6, beyond which irreversible damage occurs. The immature gut is highly susceptible to IH-induced injury, and IH may contribute to pathological outcomes in the immature gut.
Impact statement
-
1.
The neonatal gut at birth is highly susceptible to intermittent hypoxia (IH) injury.
-
2.
IH causes gut dysbiosis, inflammation, and glutathione depletion.
-
3.
The severity of gut injury worsens as a function of increasing daily IH episodes.
-
4.
The critical number of daily IH episodes that the newborn gut may sustain is 6, beyond which irreversible damage occurs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Saugstad, O. D. Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatr. Res. 23, 143–150 (1988).
Perez, M., Robbins, M. E., Revhaug, C. & Saugstad, O. D. Oxygen radical disease in the newborn, revisited: oxidative stress and disease in the newborn period. Free Radic. Biol. Med. 142, 61–72 (2019).
Beharry, K. D. et al. Neonatal intermittent hypoxia, reactive oxygen species, and oxygen-induced retinopathy. React. Oxyg. Species 3, 12–25 (2017).
Di Fiore, J. M., Martin, R. J. & Gauda, E. B. Apnea of prematurity-perfect storm. Respir. Physiol. Neurobiol. 189, 213–222 (2013).
Ford, H., Watkins, S., Reblock, K. & Rowe, M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J. Pediatr. Surg. 32, 275–282 (1997).
Wohlrab, P. et al. Oxygen conditions oscillating between hypoxia and hyperoxia induce different effects in the pulmonary endothelium compared to constant oxygen conditions. Physiol. Rep. 9, e14590 (2019).
Ray, P. D., Huang, B. W. & Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24, 981–990 (2012).
Aceti, A., Beghetti, I., Martini, S., Faldella, G. & Corvaglia, L. Oxidative stress and necrotizing enterocolitis: pathogenetic mechanisms, opportunities for intervention, and role of human milk. Oxid. Med. Cell Longev. 2018, 7397659 (2018).
Schnabl, K., Van Aerde, J. E., Thomson, A. B. & Clandinin, M. T. Necrotizing enterocolitis: a multifactorial disease with no cure. World J. Gastroenterol. 14, 2142–2161 (2008).
Yee, W. H. et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 129, e298–e304 (2012).
Zozaya, C. et al. Incidence, treatment, and outcome trends of necrotizing enterocolitis in preterm infants: a multicenter cohort study. Front. Pediatr. 8, 188 (2020).
Nino, D. F., Sodhi, C. P. & Hackam, D. J. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 13, 590–600 (2016).
Leaphart, C. L. et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J. Immunol. 179, 4808–4820 (2007).
Claud, E. Neonatal necrotizing enterocolitis–inflammation and intestinal immaturity. Antiinflamm. Antiallergy Agents Med. Chem. 8, 248–259 (2009).
Hosny, M., Cassir, N. & La Scola, B. Updating on gut microbiota and its relationship with the occurrence of necrotizing enterocolitis. Hum. Microb. J. 4, 14–19 (2017).
Habbout, A., Li, N., Rochette, L. & Vergely, C. Postnatal overfeeding in rodents by litter size reduction induces major short- and long-term pathophysiological consequences. J. Nutr. 143, 553–562 (2013).
Parra-Vargas, M., Ramon-Krauel, M., Lerin, C. & Jimenez-Chillaron, J. C. Size does matter: litter size strongly determines adult metabolism in rodents. Cell Metab. 32, 334–340 (2020).
Beharry, K. D. et al. Hydrogen peroxide accumulation in the choroid during intermittent hypoxia increases risk of severe oxygen-induced retinopathy in neonatal rats. Investig. Ophthalmol. Vis. Sci. 54, 7644–7657 (2013).
Coleman, R. et al. Effects of brief clustered versus dispersed hypoxiv episodes on systemic and ocular growth factors in a rat model of OIR. Pediatr. Res. 64, 50–55 (2008).
Bodkin, D. et al. Neonatal intermittent hypoxia, fish oil, and/or antioxidant supplementation on gut microbiota in neonatal rats. Pediatr. Res 92, 109–117 (2022).
Cuna, A., Morowitz, M. J., Ahmed, I., Umar, S. & Sampath, V. Dynamics of the preterm gut microbiome in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 320, G411–G419 (2021).
Puiman, P. & Stoll, B. Animal models to study neonatal nutrition in humans. Curr. Opin. Clin. Nutr. Metab. Care 11, 601–606 (2008).
McCracken, V. J. & Lorenz, R. G. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 3, 1–11 (2001).
Wu, J. et al. Disrupted intestinal structure in a rat model of intermittent hypoxia. Mol. Med. Rep. 13, 4407–4413 (2016).
Xing, J. et al. Hypoxia induces senescence of bone marrow mesenchymal stem cells via altered gut microbiota. Nat. Commun. 9, 2020 (2018).
Xu, C. L. et al. Protective effect of glutamine on intestinal injury and bacterial community in rats exposed to hypobaric hypoxia environment. World J. Gastroenterol. 20, 4662–4674 (2014).
Kaplina, A. et al. Necrotizing enterocolitis: the role of hypoxia, gut microbiome, and microbial metabolites. Int J. Mol. Sci. 24, 2471 (2023).
Martin, C. R., Osadchiy, V., Kalani, A. & Mayer, E. A. The brain-gut-microbiome axis. Cell Mol. Gastroenterol. Hepatol. 6, 133–148 (2018).
Tripathi, A. et al. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 15, 397–411 (2018).
Dang, A. T. & Marsland, B. J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 12, 843–850 (2019).
Korpela, K. et al. Intestinal microbiota development and gestational age in preterm neonates. Sci. Rep. 8, 2453 (2018).
Mai, V. et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One 6, e20647 (2011).
Wilson, A., Bogie, B., Chaaban, H. & Burge, K. The nonbacterial microbiome: fungal and viral contributions to the preterm infant gut in health and disease. Microorganisms 11, 909 (2023).
Rinninella, E. et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7, 14 (2019).
Di Fiore, J. M. & Vento, M. Intermittent hypoxemia and oxidative stress in preterm infants. Respir. Physiol. Neurobiol. 266, 121–129 (2019).
Denning, N. L. & Prince, J. M. Neonatal intestinal dysbiosis in necrotizing enterocolitis. Mol. Med. 24, 4 (2018).
Pammi, M. et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5, 31 (2017).
Han, N., Pan, Z., Liu, G., Yang, R. & Yujing, B. Hypoxia: the “Invisible Pusher” of gut microbiota. Front. Microbiol. 12, 690600 (2021).
Li, Z. P. et al. Overgrowth of Lactobacillus in gastric cancer. World J. Gastrointest. Oncol. 13, 1099–1108 (2021).
Wang, Y. et al. Lactobacillus reuteri in its biofilm state promotes neurodevelopment after experimental necrotizing enterocolitis in rats. Brain Behav. Immun. Health 14, 100256 (2021).
Yu, W. et al. SIGIRR mutation in human necrotizing enterocolitis (NEC) disrupts STAT3-dependent microRNA expression in neonatal gut. Cell Mol. Gastroenterol. Hepatol. 13, 425–440 (2022).
Hackam, D. J., Afrazi, A., Good, M. & Sodhi, C. P. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clin. Dev. Immunol. 2013, 475415 (2013).
Vyas, D. et al. Epithelial apoptosis in mechanistically distinct methods of injury in the murine small intestine. Histol. Histopathol. 22, 623–630 (2007).
Ikeda, H. et al. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut 42, 530–537 (1998).
Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 33, 127–148 (2021).
Schaefer, E. et al. Intermittent hypoxia is a proinflammatory stimulus resulting in IL-6 expression and M1 macrophage polarization. Hepatol. Commun. 1, 326–337 (2017).
Chen, L. et al. Severe intermittent hypoxia modulates the macrophage phenotype and impairs wound healing through downregulation of HIF-2α. Nat. Sci. Sleep 14, 1511–1520 (2022).
Almonaem, E. R. A., Almotaleb, G. S. A., Alhameed, M. H. A. & El-Shimi, O. S. Utility of transforming growth factor beta-1 in diagnosis of neonatal necrotizing enterocolitis. J. Neonatal. Perinat. Med. 15, 795–801 (2022).
Zhang, X. et al. β-glucan protects against necrotizing enterocolitis in mice by inhibiting intestinal inflammation, improving the gut barrier, and modulating gut microbiota. J. Transl. Med. 21, 14 (2023).
Li, J. et al. Ferroptosis: past, present and future. Cell Death Dis. 11, 88 (2020).
Bensouda, B., Tarazi, S. E., Ali, N., Mandel, R. & Sant’Anna, G. M. Episodes of apnea, desaturation and bradycardia and the development of necrotizing enterocolitis in preterm infants: a case-control study. J. Matern Fetal Neonatal. Med. 26, 52–55 (2013).
Thänert, R., Keen, E. C., Dantas, G., Warner, B. B. & Tarr, P. I. Necrotizing enterocolitis and the microbiome: current status and future directions. J. Infect. Dis. 223, S257–S263 (2021).
Acknowledgements
This work was made possible through the Eunice Kennedy Shriver National Institute of Child Health & Human Development Grant #U54HD071594.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the conception (M.L., J.V.A., K.D.B.), methodology and design of the work (M.L., J.V.A., K.D.B.), investigation (M.L., C.L.C., M.M., M.M., K.D.B.), acquisition and formal analysis (M.L., C.L.C., M.M., M.M., K.D.B.), validation (M.L., J.V.A., K.D.B.), data curation (M.L., K.D.B.), interpretation of the data (M.L., J.V.A., K.D.B.), visualization (M.L., C.L.C., M.M., M.M., K.D.B.), original draft (M.L.), and review and approval of the submitted version (M.L., C.L.C., M.M., M.M., J.V.A., K.D.B.), supervision (J.V.A., K.D.B.), project administration (J.V.A., K.D.B.), funding acquisition (J.V.A.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Latkowska, M., Cai, C.L., Mitrou, M. et al. Gut microbiome and inflammation in response to increasing intermittent hypoxia in the neonatal rat. Pediatr Res 97, 2126–2135 (2025). https://doi.org/10.1038/s41390-024-03569-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03569-7