Abstract
Background
Bronchopulmonary dysplasia (BPD) is a chronic lung disease affecting premature infants who require oxygen supplementation and ventilator therapy to support their underdeveloped lungs. Autotaxin (ATX), an enzyme that generates the bioactive phospholipid lysophosphatidic acid (LPA), which acts via G-protein coupled receptors, has been implicated in numerous pulmonary diseases. In this study, we explored the pathophysiological role of the ATX/LPA signaling pathway in BPD.
Methods
Neonatal mice were exposed to normoxia or hyperoxia (85%) for 14 days from birth while being treated with vehicle, ATX inhibitor or LPA receptor 1 (LPA1) inhibitor. In vitro studies utilized human lung fibroblast (HLF) cells exposed to room air, 85% oxygen, or LPA for varying time periods. Supernatants and cells were collected for assays and Western blotting.
Results
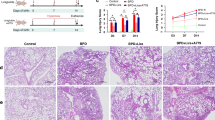
Animals exposed to hyperoxia showed elevated expression of ATX, ATX activity, and LPA1. Inhibiting ATX or LPA1 improved alveolarization, reduced inflammation, and mitigated extracellular matrix deposition and lysyl oxidase (LOX) expression. LPA1 inhibition leading to reduced LOX expression was associated with a reduction in phosphorylation of AKT.
Conclusion
Hyperoxia increases the expression of ATX and LPA1 associated with increased LOX in the lungs. Targeting the ATX/LPA1 pathway could be a potential therapeutic approach to BPD.
Impact
-
Exposure to hyperoxia increases the expression and activity of autotaxin (ATX), as well as expression of LPA receptor 1 (LPA1).
-
Increased expression of ATX influences extra cellular matrix (ECM) remodeling.
-
Inhibitors targeting the ATX/LPA pathway could offer a new therapeutic approach to bronchopulmonary dysplasia (BPD), potentially mitigating ECM deposition and improving lung development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout













Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the published article along with the supplementary information files. No other data sets were generated or analyzed.
References
Hintz, S. R. et al. Predicting time to hospital discharge for extremely preterm infants. Pediatrics 125, 146–154 (2010).
Stoll, B. J. et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics 126, 443–156 (2010).
Thekkeveedu, R. K., Guaman, M. C. & Shivanna, B. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Resp. Med. 132, 170–177 (2017).
Bonikos, D. S., Bensch, K. G. & Northway, W. H. Oxygen toxicity in the newborn. The effect of chronic continuous 100 percent oxygen exposure on the lungs of newborn mice. Am. J. Pathol. 85, 623–650 (1976).
Dreyfuss, D. & Saumon, G. Ventilator-induced Lung Injury. Am. J. Resp. Crit. Care 157, 294–323 (1998).
Pasha, A. B., Chen, X.-Q. & Zhou, G.-P. Bronchopulmonary dysplasia: Pathognesis and treatment. Exp. Ther. Med. 16, 4315–4321 (2018).
Hennessy, E. M. et al. Respiratory health in pre-school and school age children following extremely preterm birth. Arch. Dis. Child. 93, 1037 (2008).
Shahzad, T., Radajewski, S., Chao, C.-M., Bellusci, S. & Ehrhardt, H. Pathogenesis of bronchopulmonary dysplasia: when inflammation meets organ development. Mol. Cell Pediatrics 3, 23 (2016).
Thibeault, D. W., Mabry, S. M., Ekekezie, I. I., Zhang, X. & Truog, W. E. Collagen Scaffolding During Development and Its Deformation With Chronic Lung Disease. Pediatrics 111, 766–776 (2003).
Pierce, R. A. et al. Chronic lung injury in preterm lambs: disordered pulmonary elastin deposition. Am. J. Physiol. Lung 272, L452–L460 (1997).
Kumarasamy, A. et al. Lysyl oxidase activity is dysregulated during impaired alveolarization of mouse and human lungs. Am. J. Resp. Crit. Care 180, 1239–1252 (2009).
Ha, A. W. et al. Sphingosine kinase 1 regulates lysyl oxidase through STAT3 in hyperoxia-mediated neonatal lung injury. Thorax 77, 47–57 (2022).
Li, H. et al. Association between plasma lysophosphatidic acid levels and bronchopulmonary dysplasia in extremely preterm infants: A prospective study. Pediatr. Pulmonol. 58, 3516–3522 (2023).
Umezu-Goto, M. et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 158, 227–233 (2002).
Boutin, J. A. & Ferry, G. Autotaxin. Cell. Mol. Life Sci. 66, 3009 (2009).
Croset, M., Brossard, N., Polette, A. & Lagarde, M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem. J. 345, 61 (2000).
Moolenaar, W. H. Development of Our Current Understanding of Bioactive Lysophospholipids. Ann. Ny. Acad. Sci. 905, 1–10 (2000).
Stortelers, C., Kerkhoven, R. & Moolenaar, W. H. Multiple actions of lysophosphatidic acid on fibroblasts revealed by transcriptional profiling. BMC Genomics 9, 387 (2008).
Asokan, S. B. et al. Lysophosphatidic acid provokes fibroblast chemotaxis through combinatorial regulation of myosin II. Biorxiv 355610 (2019).
Toews, M. L., Ediger, T. L., Romberger, D. J. & Rennard, S. I. Lysophosphatidic acid in airway function and disease. Biochimica Et. Biophysica Acta Bba – Mol. Cell. Biol. Lipids 1582, 240–250 (2002).
Hayashi, K. et al. Phenotypic Modulation of Vascular Smooth Muscle Cells Induced by Unsaturated Lysophosphatidic Acids. Circ. Res. 89, 251–258 (2001).
Tager, A. M. et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 14, 45–54 (2008).
Park, G. Y. et al. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am. J. Resp. Crit. Care Med. 188, 928–940 (2013).
Li, Q. et al. Lysophosphatidic acid species are associated with exacerbation in chronic obstructive pulmonary disease. BMC Pulm. Med. 21, 301 (2021).
Mouratis, M.-A. et al. Autotaxin and Endotoxin-Induced Acute Lung Injury. Plos One 10, e0133619 (2015).
Shim, G. H. et al. Expression of autotaxin and lysophosphatidic acid receptors 1 and 3 in the developing rat lung and in response to hyperoxia. Free Radic. Res 49, 1362–1370 (2015).
Chen, X. et al. Deficiency or inhibition of lysophosphatidic acid receptor 1 protects against hyperoxia‐induced lung injury in neonatal rats. Acta Physiol. 216, 358–375 (2016).
Ha, A. W. et al. Neonatal therapy with PF543, a sphingosine kinase 1 inhibitor, ameliorates hyperoxia-induced airway remodeling in a murine model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 319, L497–L512 (2020).
Harijith, A. et al. Hyperoxia-induced p47phox activation and ROS generation is mediated through S1P transporter Spns2, and S1P/S1P1&2 signaling axis in lung endothelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 311, L337–L351 (2016).
Tang, X. et al. Inhibition of autotaxin with GLPG1690 increases the efficacy of radiotherapy and chemotherapy in a mouse model of breast cancer. Mol. Cancer Ther. 19, 63–74 (2020).
Laurent, G. J. Collagen in normal lung and during pulmonary fibrosis. Cellular Biology of the Lung 311-325 (1982).
Zandvoort, A. et al. Smad gene expression in pulmonary fibroblasts: indications for defective ECM repair in COPD. Respir. Res. 9, 83–83 (2008).
Wei, S., Gao, L., Wu, C., Qin, F. & Yuan, J. Role of the lysyl oxidase family in organ development (Review). Exp. Ther. Med. 20, 163–172 (2020).
Cheng, P. T. W. et al. Discovery of an oxcyclohexyl acid lysophosphatidic acid receptor 1 (LPA1) antagonist BMS-986278 for the treatment of pulmonary fibrotic diseases. J. Med. Chem. 64, 15549–15581 (2020).
Suryadevara, V., Ramchandran, R., Kamp, D. W. & Natarajan, V. Lipid mediators regulate pulmonary fibrosis: potential mechanisms and signaling pathways. Int. J. Mol. Sci. 21, 4257 (2020).
Tager, A. M. Autotaxin emerges as a therapeutic target for idiopathic pulmonary fibrosis: limiting fibrosis by limiting lysophosphatidic acid synthesis. Am. J. Resp. Cell Mol. 47, 563–565 (2012).
Ninou, I., Magkrioti, C. & Aidinis, V. Autotaxin in pathophysiology and pulmonary fibrosis. Front. Med. 5, 180 (2018).
Gao, L. et al. Autotaxin levels in serum and bronchoalveolar lavage fluid are associated with inflammatory and fibrotic biomarkers and the clinical outcome in patients with acute respiratory distress syndrome. J. Intensive Care 9, 44 (2021).
Perrakis, A. & Moolenaar, W. H. Autotaxin: structure-function and signaling. J. Lipid Res. 55, 1010–1018 (2014).
Clark, J. M. et al. Structure-based design of a novel class of autotaxin inhibitors based on endogenous allosteric modulators. J. Med. Chem. 65, 6338–6351 (2022).
Geraldo, L. H. M. et al. Role of lysophosphatidic acid and its receptors in health and disease: novel therapeutic strategies. Signal Transduct. Target Ther. 6, 45 (2021).
Salgado-Polo, F. & Perrakis, A. The structural binding mode of the four autotaxin inhibitor types that differentially affect catalytic and non-catalytic functions. Cancers 11, 1577 (2019).
Black, K. E. et al. Autotaxin activity increases locally following lung injury, but is not required for pulmonary lysophosphatidic acid production or fibrosis. Faseb J. 30, 2435–2450 (2016).
Castelino, F. V. et al. An autotaxin/lysophosphatidic acid/interleukin‐6 amplification loop drives scleroderma fibrosis. Arthritis Rheumatol. 68, 2964–2974 (2016).
Benesch, M. G. K. et al. Autotaxin is an inflammatory mediator and therapeutic target in thyroid cancer. Endocr.-relat. Cancer 22, 593–607 (2015).
Voloshenyuk, T. G., Fournett, A. C. & Gardner, J. D. PI3K/Akt signaling mediates increased BMP‐1 expression in response to TNF‐α and TGF‐β1 in cardiac fibroblasts. Faseb J. 25, 1032.3 (2011).
Oikonomou, N. et al. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am. J. Resp. Cell Mol. 47, 566–574 (2012).
Piersigilli, F. & Bhandari, V. Metabolomics of bronchopulmonary dysplasia. Clin. Chim. Acta 500, 109–114 (2020).
Frano, M. R. L. et al. Umbilical cord blood metabolomics reveal distinct signatures of dyslipidemia prior to bronchopulmonary dysplasia and pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 315, L870–L881 (2018).
Baraldi, E. et al. Untargeted metabolomic analysis of amniotic fluid in the prediction of preterm delivery and bronchopulmonary dysplasia. Plos One 11, e0164211 (2016).
Carraro, S. et al. Airway metabolic anomalies in adolescents with bronchopulmonary dysplasia: new insights from the metabolomic approach. J. Pediatrics 166, 234–239.e1 (2015).
Wheelock, C. E. et al. Application of ’omics technologies to biomarker discovery in inflammatory lung diseases. Eur. Respir. J. 42, 802–825 (2013).
Ojala, P. J., Hirvonen, T. E., Hermansson, M., Somerharju, P. & Parkkinen, J. Acyl chain‐dependent effect of lysophosphatidylcholine on human neutrophils. J. Leukoc. Biol. 82, 1501–1509 (2007).
Azare, J. et al. Stat3 Mediates Expression of Autotaxin in Breast Cancer. Plos One 6, e27851 (2011).
Sureshbabu, A. et al. Conditional overexpression of TGFβ1 promotes pulmonary inflammation, apoptosis and mortality via TGFβR2 in the developing mouse lung. Respir. Res 16, 4 (2015).
Calthorpe, R. J. et al. Complex roles of TGF-β signaling pathways in lung development and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 324, L285–L296 (2023).
Xu, M. Y. et al. Lysophosphatidic acid induces αvβ6 integrin-mediated TGF-β activation via the LPA2 receptor and the small G protein Gαq. Am. J. Pathol. 174, 1264–1279 (2009).
Nathan, S., Zhang, H., Andreoli, M., Leopold, P. L. & Crystal, R. G. CREB-dependent LPA-induced signaling initiates a pro-fibrotic feedback loop between small airway basal cells and fibroblasts. Respir. Res. 22, 97 (2021).
Huang, L. S. et al. Lysophosphatidic acid receptor–2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice. Am. J. Resp. Cell Mol. 49, 912–922 (2013).
Igarashi, N. et al. Crosstalk between transforming growth factor β-2 and Autotaxin in trabecular meshwork and different subtypes of glaucoma. J. Biomed. Sci. 48, 47 (2021).
Mizikova, I. & Morty, R. E. The extracellular matrix in bronchopulmonary dysplasia: target and source. Front. Med. 2, 91 (2015).
Funke, M., Zhao, Z., Xu, Y., Chun, J. & Tager, A. M. The lysophosphatidic acid receptor LPA1 promotes epithelial cell apoptosis after lung injury. Am. J. Resp. Cell Mol. 46, 355–364 (2012).
Acknowledgements
The authors would like to thank Dr. Xiaoyun Tang and Dr. David N. Brindley from the University of Alberta for sharing their ATX activity assay protocol, as well as providing valuable technical support. The authors would also like to thank David Fletcher and Dr. Tracey Bonfield from Case Western Reserve University’s Bioanalyte Core for their assistance with the Mouse Luminex Discovery Assay. Lastly, the authors would like to thank University Hospitals Rainbow Babies and Children’s Hospital in Cleveland for providing the lung tissue samples.
Funding
This work was supported in part by #18TPA34230095/American Heart Association and R01HD090887/Eunice Kennedy Shriver National Institute of Child Health and Human Development to A.H.
Author information
Authors and Affiliations
Contributions
A.W.H., V.N., and A.H. conceived, and designed research; A.W.H, T.S, A.J., C.M., and A.H. performed experiments; A.W.H., P.M.M., V.N., and A.H. analyzed data and interpreted results of experiments; A.W.H. and A.H. drafted manuscript; A.W.H., V.N., and A.H. edited and revised manuscript; All authors read and approved final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ha, A.W., Sudhadevi, T., Jafri, A. et al. Bronchopulmonary dysplasia demonstrates dysregulated autotaxin/lysophosphatidic acid signaling in a neonatal mouse model. Pediatr Res 97, 2462–2474 (2025). https://doi.org/10.1038/s41390-024-03610-9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03610-9