Abstract
Background
In this study, we aimed to explore the role of Tarm1 in juvenile mice with dextran sulfate sodium (DSS)-induced colitis and elucidate the mechanisms that affect intestinal barrier function.
Methods
A DSS-induced pediatric inflammatory bowel disease mouse model was established using 4-week-old juvenile mice. Disease activity index and histopathological damage scores were determined using hematoxylin and eosin (H&E) staining. Tarm1, F4/80, CD68, and CD86 levels were detected using qPCR, western blotting, and immunofluorescence. Trans epithelial electric resistance (TEER) was detected using the transwell assay.
Results
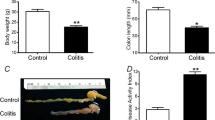
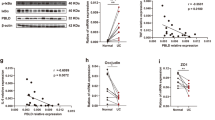
Results revealed that juvenile colitis mice fed 4% DSS drinking water had increased Tarm1 expression in the colon tissue, increased macrophage M1 polarization, higher expression of pro-inflammatory cytokines, and an impaired intestinal mucosal barrier, compared with the control group. Tarm1-knockdown RAW264.7 cells inhibited lipopolysaccharide (LPS)-induced M1 polarization and attenuated barrier damage in co-cultured intestinal epithelial cells.
Conclusion
Tarm1 expression was increased in colonic tissues of juvenile mice with colitis, and LPS-induced M1 polarization and intestinal barrier damage were attenuated in Tarm1-knockdown RAW264.7 cells. This suggests that attenuation of Tarm1 expression is a potential target for pediatric inflammatory bowel disease therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Jans, D. & Cleynen, I. The genetics of non-monogenic IBD. Hum. Genet. 142, 669–682 (2023).
Wang, X. Q. et al. Inflammatory bowel disease in Chinese children: a multicenter analysis over a decade from Shanghai. Inflamm. Bowel Dis. 19, 423–428 (2013).
Kudo, T. et al. Very early-onset inflammatory bowel disease in Japan: a nationwide survey. J. Gastroenterol. Hepatol. 36, 151–155 (2021).
Spalinger, M. R. et al. PTPN2 regulates interactions between macrophages and intestinal epithelial cells to promote intestinal barrier function. Gastroenterology 159, 1763–77 e14 (2020).
Na, Y. R., Stakenborg, M., Seok, S. H. & Matteoli, G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 16, 531–543 (2019).
Gerner, R. R. et al. NAD metabolism fuels human and mouse intestinal inflammation. Gut 67, 1813–1823 (2018).
Bain, C. C. et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6, 498–510 (2013).
Delfini, M., Stakenborg, N., Viola, M. F. & Boeckxstaens, G. Macrophages in the gut: masters in multitasking. Immunity 55, 1530–1548 (2022).
Viola, M. F. & Boeckxstaens, G. Niche-specific functional heterogeneity of intestinal resident macrophages. Gut. 70, 1383–1395 (2021).
Castro-Dopico, T. et al. GM-CSF calibrates macrophage defense and wound healing programs during intestinal infection and inflammation. Cell Rep. 32, 107857 (2020).
Lissner, D. et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm. Bowel Dis. 21, 1297–1305 (2015).
Zhou, X. et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. 27, 1176–89 e5 (2019).
Pull, S. L., Doherty, J. M., Mills, J. C., Gordon, J. I. & Stappenbeck, T. S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl Acad. Sci. USA 102, 99–104 (2005).
Guo, J., Zhang, Y. Y., Sun, M. & Xu, L. F. Therapeutic potential of curcumin in a rat model of dextran sulfate sodium-induced ulcerative colitis by regulating the balance of Treg/Th17 cells. Inflammation 45, 2163–2171 (2022).
Li, G. et al. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 13, 1968257 (2021).
Guo, F. et al. Polyphenol Content of Green Pea (Pisum sativum L.) hull under in vitro digestion and effects of digestive products on anti-inflammatory activity and intestinal barrier in the Caco-2/Raw264.7 Coculture Model. J. Agric Food Chem. 70, 3477–3488 (2022).
Hegarty, L. M., Jones, G. R. & Bain, C. C. Macrophages in intestinal homeostasis and inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 20, 538–553 (2023).
Hua, H. et al. Probiotic lactic acid bacteria alleviate pediatric IBD and remodel gut microbiota by modulating macrophage polarization and suppressing epithelial apoptosis. Front. Microbiol. 14, 1168924 (2023).
Sommer, K. et al. Intestinal mucosal wound healing and barrier integrity in IBD-Crosstalk and trafficking of cellular players. Front. Med. 8, 643973 (2021).
Al-Ghadban, S., Kaissi, S., Homaidan, F. R., Naim, H. Y. & El-Sabban, M. E. Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease. Sci. Rep. 6, 29783 (2016).
De Sablet, T. et al. Cryptosporidium parvum increases intestinal permeability through interaction with epithelial cells and IL-1beta and TNFalpha released by inflammatory monocytes. Cell Microbiol. 18, 1871–1880 (2016).
Yabe, R. et al. TARM1 contributes to development of arthritis by activating dendritic cells through recognition of collagens. Nat. Commun. 12, 94 (2021).
Radjabova, V. et al. TARM1 is a novel leukocyte receptor complex-encoded ITAM receptor that costimulates proinflammatory cytokine secretion by macrophages and neutrophils. J. Immunol. 195, 3149–3159 (2015).
Li, X. et al. TARM-1 is critical for macrophage activation and Th1 response in mycobacterium tuberculosis infection. J. Immunol. 207, 234–243 (2021).
Funding
This work was supported by the [Basic Research Projects of Liaoning Provincial Department of Education in 2022] under Grant [LJKMZ20221184] from Jing Guo, National Key Research and Development Program of China [2023YFC2706503] and 345 Talent Project from Lingfen Xu.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Author Kun Zhang had the primary responsibility for animal experiments, cell experiments, outcome assessment, preliminary data analysis and drafting the manuscript. Author Lingfen Xu revised the article critically for important intellectual content. Author Jing Guo as a communication author contributed to the writing of the manuscript and the overall guidance. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors read and approved the final manuscript.
Ethical approval
This study was approved by the Ethics Committee of the Affiliated Shengjing Hospital of the China Medical University (approval no. 2023PS203K; Shenyang, China).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, K., Xu, L. & Guo, J. Tarm1 may affect colitis by regulating macrophage M1 polarization in a mouse colitis model. Pediatr Res 98, 286–293 (2025). https://doi.org/10.1038/s41390-024-03640-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03640-3
This article is cited by
-
Ginseng exosomes modulate M1/M2 polarisation by activating autophagy and target IKK/IкB/NF-кB to alleviate inflammatory bowel disease
Journal of Nanobiotechnology (2025)