Abstract
Background
While the incidence of neonatal intensive care unit (NICU) admission steadily increases, neonatology lacks evidence of a safe, effective, and preventive analgesic for treating procedural pain. Given its role in nociception and promoting healthy neurodevelopment, the endogenous neuropeptide oxytocin (OT) emerges as a promising candidate.
Methods
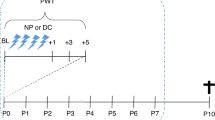
This study investigates the use of daily repeated subcutaneous OT (1 mg/kg) treatment in an established model of neonatal repetitive procedural pain and assesses the effectivity of OT treatment on mechanical sensitivity and body weight.
Results
Contrary to our hypothesis repeated daily OT treatment did not prevent the development of mechanical hypersensitivity following needle pricks. Furthermore, treatment with OT diminished body weight gain in neonatal pups, a major side effect observed throughout the neonatal week. These results highlight the unique nature of the maturing nociceptive system that makes the identification and selection of analgesic options for the treatment of acute neonatal procedural pain a major challenge.
Conclusion
In conclusion, our preclinical results do not support the use of repeated OT for acute pain relief in the NICU, and the side effects on body weight gain raise concerns about the use of OT in the NICU.
Impact
-
Repeated daily OT treatment inhibits weight gain in neonatal rat pus.
-
Repetitive daily OT administration does not prevent the development of mechanical hypersensitivity in a model of neonatal procedural pain.
-
Future research must focus on the unique physiology of the developing nociceptive system to establish safe, effective and protective treatment of neonatal procedural pain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Blencowe, H. et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod. Health 10, 1–14 (2013).
Tielsch J. M. Global incidence of preterm birth. In: Low-Birthweight Baby: Born Too Soon or Too Small. Karger Publishers. 9–15 (2015).
Gamber, R. A., Blonsky, H., Mcdowell, M. & Lakshminrusimha, S. Declining birth rates, increasing maternal age and neonatal intensive care unit admissions. J. Perinatol. 44, 203–208 (2024).
Cruz, M. D., Fernandes, A. M. & Oliveira, C. R. Epidemiology of painful procedures performed in neonates: A systematic review of observational studies. Eur. J. Pain. 20, 489–498 (2016).
Carbajal, R. et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 300, 60–70 (2008).
Roofthooft, D. W. E., Simons, S. H. P., Anand, K. J. S., Tibboel, D. & Van Dijk, M. Eight years later, are we still hurting newborn infants? Neonatology 105, 218–226 (2014).
Simons, S. H. P. et al. Do we still hurt newborn babies? a prospective study of procedural pain and analgesia in neonates. Arch. Pediatr. Adolesc. Med. 157, 1058–1064 (2003).
Claudia, Y. P. A. et al. Painful procedures and analgesia in the NICU: what has changed in the medical perception and practice in a ten-year period? J. Pediatr. 92, 88–95 (2016).
Hartley, C. et al. Analgesic efficacy and safety of morphine in the Procedural Pain in Premature Infants (Poppi) study: Randomised placebo-controlled trial. Lancet 392, 2595 (2018).
Hartley, C. et al. Predicting severity of adverse cardiorespiratory effects of morphine in premature infants: a post hoc analysis of Procedural Pain in Premature Infants trial data. Br. J. Anaesth. 126, 133–154 (2021).
Allegaert, K., Bellieni, C. & Guimarães, H. A critical review on the relevance of paracetamol for procedural pain management in neonates. Front Pediatr. 8, 89 (2020).
Squillaro, A. et al. Managing procedural pain in the neonate using an opioid-sparing approach. Clin. Ther. 41, 1701–1713 (2019).
Zwicker, J. G. et al. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. J. Pediatr. 172, 81–87.e2 (2016).
Attarian, S. et al. The neurodevelopmental impact of neonatal morphine administration. Brain Sci. 4, 321–334 (2014).
World Health Organization. Cancer pain relief. Geneva: World Health Organization; (1986).
Carbajal, R. et al. Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics 115, 1494–1500 (2005).
Breton J.-D. et al. Oxytocin-induced antinociception in the spinal cord is mediated by a subpopulation of glutamatergic neurons in lamina I-II which amplify GABAergic inhibition. Mol. Pain 4, https://doi.org/10.1186/1744-8069-4-19 (2004).
Millan, M. J. Descending control of pain. Prog. Neurobiol. 66, 355–474 (2002).
Juif, P. E. & Poisbeau, P. Neurohormonal effects of oxytocin and vasopressin receptor agonists on spinal pain processing in male rats. Pain 154, 1449–1456 (2013).
Poisbeau P., Grinevich V., Charlet A. Oxytocin signaling in pain: cellular, circuit, system, and behavioral levels. Behavioral pharmacology of neuropeptides: oxytocin. 193–211. (2018).
Leonzino, M. et al. The timing of the excitatory-to-inhibitory GABA switch is regulated by the oxytocin receptor via KCC2. Cell Rep. 15, 96–103 (2016).
Li Y.-X., An H., Wen Z., Tao Z.-Y., Cao D.-Y. Can oxytocin inhibit stress-induced hyperalgesia? Neuropeptides 79, 101996 (2019).
Onaka, T. & Takayanagi, Y. The oxytocin system and early‐life experience‐dependent plastic changes. J. Neuroendocrinol. 33, e13049 (2021).
Godínez-Chaparro B. et al. The potential role of serotonergic mechanisms in the spinal oxytocin-induced antinociception. Neuropeptides 60, 51–60 (2016).
Filippa, M. et al. Pain, parental involvement, and oxytocin in the neonatal intensive care unit. Front Psychol. 10, 715 (2019).
de Kort, A. R., Joosten, E. A., Patijn, J., Tibboel, D. & van den Hoogen, N. J. Anatomical changes in descending serotonergic projections from the rostral ventromedial medulla to the spinal dorsal horn following repetitive neonatal painful procedures. Int. J. Dev. Neurosci. 82, 361–371 (2022).
Filippa, M. et al. Maternal speech decreases pain scores and increases oxytocin levels in preterm infants during painful procedures. Sci. Rep. 11, 17301 (2021).
Anagnostou, E. et al. Intranasal oxytocin in the treatment of autism spectrum disorders: A review of literature and early safety and efficacy data in youth. Brain Res. 1580, 188–198 (2014).
Verhees, M. W. F. T. et al. No side-effects of single intranasal oxytocin administration in middle childhood. Psychopharmacology 235, 2471–2477 (2018).
Hermesch, A. C., Kernberg, A. S., Layoun, V. R. & Caughey, A. B. Oxytocin: Physiology, pharmacology, and clinical application for labor management. Am. J. Obstet. Gynecol. 230, S729–S7391500 (2024).
de Kort, A. R., Joosten, E. A., Patijn, J., Tibboel, D. & van den Hoogen, N. J. Neonatal procedural pain affects state, but not trait anxiety behavior in adult rats. Dev. Psychobiol. 63, e22210 (2021).
de Kort, A. R., Joosten, E. A., Patijn, J., Tibboel, D. & van den Hoogen, N. J. Selective targeting of serotonin 5-HT1a and 5-HT3 receptors attenuates acute and long-term hypersensitivity associated with neonatal procedural pain. Front. Pain. Res. 3, 872587 (2022).
van den Hoogen, N. J., Patijn, J., Tibboel, D. & Joosten, E. A. Repetitive noxious stimuli during early development affect acute and long-term mechanical sensitivity in rats. Pediatr. Res. 87, 26–31 (2020).
van den Hoogen, N. J., van Reij, R. R. I., Patijn, J., Tibboel, D. & Joosten, E. A. J. Adult spinal opioid receptor μ1 expression after incision is altered by early life repetitive tactile and noxious procedures in rats. Dev. Neurobiol. 78, 417–426 (2018).
van den Hoogen, N. J. et al. Neonatal paracetamol treatment reduces long-term nociceptive behaviour after neonatal procedural pain in rats. Eur. J. Pain. 20, 1309–1318 (2016).
Baudat, M., Simons, S. H. P. & Joosten, E. A. J. Repetitive neonatal procedural pain affects stress-induced plasma corticosterone increase in young adult females but not in male rats. Dev Psychobiol. 66, e22478 (2024).
Knaepen, L. et al. Neonatal repetitive needle pricking: plasticity of the spinal nociceptive circuit and extended postoperative pain in later life. Dev. Neurobiol. 73, 85–97 (2013).
Olausson, H., Uvnäs-Moberg, K. & Sohlström, A. Postnatal oxytocin alleviates adverse effects in adult rat offspring caused by maternal malnutrition. Am. J. Physiol. Endocrunol Metab. 284, 475–480 (2003).
Sohlström, A., Olausson, H., Brismar, K. & Uvnäs-Moberg, K. Oxytocin treatment during early life influences reproductive performance in ad libitum fed and food-restricted female rats. Biol. Neonate 81, 132–138 (2002).
Holst, S., Uvnäs-Moberg, K. & Petersson, M. Postnatal oxytocin treatment and postnatal stroking of rats reduce blood pressure in adulthood. Autonomic Neurosci.: Basic Clin. 99, 85–90 (2002).
de Castro, V. L. S. S., Destefani, C. R., Diniz, C. & Poli, P. Evaluation of neurodevelopmental effects on rats exposed prenatally to sulfentrazone. Neurotoxicology 28, 1249–1259 (2007).
Rüedi-Bettschen, D. & Platt, D. M. Detrimental effects of self-administered methamphetamine during pregnancy on offspring development in the rat. Drug Alcohol Depend. 177, 171–177 (2017).
van den Hoogen, N. J. et al. Repeated touch and needle-prick stimulation in the neonatal period increases the baseline mechanical sensitivity and postinjury hypersensitivity of adult spinal sensory neurons. Pain 159, 1166–1175 (2018).
Higashida, H., Oshima, Y. & Yamamoto, Y. Oxytocin transported from the blood across the blood-brain barrier by receptor for advanced glycation end-products (RAGE) affects brain function related to social behavior. Peptides 178, 171230 (2024).
Yamamoto, Y. et al. Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun. Biol. 2, 76 (2019).
Gerasimenko M. et al. Receptor for advanced glycation end-products (RAGE) plays a critical role in retrieval behavior of mother mice at early postpartum. Physiol Behav. 235, 113395 (2021).
Mens, W. B. J., Wtter, A., Van, T. B. & Greidanus, W. Penetration of Neurohypophyseal Hormones from Plasma into Cerebrospinal Fluid (CSF)” Half-Times of Disappearance of These Neuropeptides from CSF. Brain Res. 262, 143–149 (1983).
Bakos, J., Lestanova, Z., Strbak, V., Havranek, T. & Bacova, Z. Neonatal manipulation of oxytocin prevents lipopolysaccharide-induced decrease in gene expression of growth factors in two developmental stages of the female rat. Neuropeptides 48, 281–286 (2014).
Melchior, M. et al. Pharmacological rescue of nociceptive hypersensitivity and oxytocin analgesia impairment in a rat model of neonatal maternal separation. Pain 159, 2630–2640 (2018).
Ba X. et al. Three-day continuous oxytocin infusion attenuates thermal and mechanical nociception by rescuing neuronal chloride homeostasis via upregulation KCC2 expression and function. Front Pharmacol. 13, 845018 (2022).
Hathway, G. et al. A postnatal switch in GABAergic control of spinal cutaneous reflexes. Eur. J. Neurosci. 23, 112–118 (2006).
Kwok, C. H. T., Devonshire, I. M., Bennett, A. J. & Hathway, G. J. Postnatal maturation of endogenous opioid systems within the periaqueductal grey and spinal dorsal horn of the rat. Pain 155, 168–178 (2014).
Battell, E. E., Lillywhite, A. & Hathway, G. J. The changing role of descending control of spinal nociception over postnatal development. Curr. Opin. Physiol. 11, 93–96 (2019).
Furukawa, M. et al. Neonatal maternal separation delays the GABA excitatory-to-inhibitory functional switch by inhibiting KCC2 expression. Biochem Biophys. Res Commun. 493, 1243–1249 (2017).
Hathway, G. J., Vega-Avelaira, D. & Fitzgerald, M. A critical period in the supraspinal control of pain: opioid-dependent changes in brainstem rostroventral medulla function in preadolescence. Pain 153, 775–783 (2012).
Morton G. J. et al. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am. J. Physiol. Endocrinol Metab. 302, E134–E144 (2012).
Deblon, N., Veyrat-Durebex, C., Bourgoin, L., Caillon, A. & Bussier, A.-L. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS One 6, 25565 (2011).
Maejima Y. et al. Impact of sex, fat distribution and initial body weight on oxytocin’s body weight regulation. Sci Rep. (2017).
Lawson E. A., Olszewski P. K., Weller A., Blevins J. E. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J. Neuroendocrinol. (2020).
Kerem L., Lawson E. A. The effects of oxytocin on appetite regulation, food intake and metabolism in humans. Int. J. Mol. Sci. (2021).
Elabd, C. et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells 26, 2399–2407 (2008).
Sutton, A. K. et al. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J. Neurosci. 34, 15306–15318 (2014).
Buijs, R. M. The development of vasopressin and oxytocin systems in the brain. Handb. Chem. Neuroanat. 10, 547–571 (1992).
Yamamoto, Y. et al. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience 125, 947–955 (2004).
Reiter M. K. et al. Localization of oxytocin binding sites in the thoracic and upper lumbar spinal cord of the adult and postnatal rat: A histoautoradiographic study. Eur. J. Neurosci. 6: 98–104. (1993).
Acknowledgements
The completion of this research project would not have been possible without the scientific discussions with colleagues L. Heijmans (Ph.D.), T.J. de Geus (MSc), I. Rudnick-Jansen (Ph.D.), G. Franken (Ph.D.). Contribution from D. Mulder-Jongen and D. Hermes, and members of the animal facility was also essential in the completion of this research. The authors have no conflict of interest to declare. Funding was provided by internal research support based on grants from Erasmus-Sophia Children’s Hospital Rotterdam, the Netherlands (to S. H.P. Simons) and Maastricht University, Maastricht, the Netherlands (to E.A.J. Joosten). All animal experiments were performed in accordance with the European Directive for Protection of Vertebrate Animal Use for Experimental and Other Scientific Purposes (86/609/EEC) and were approved by the Committee for Experiments on Animals, Maastricht, The Netherlands (DEC 2017-017).
Author information
Authors and Affiliations
Contributions
All authors substantially contributed to the conception and design of the experiment, interpreted the data and critically revised the manuscript. Acquisition of data and drafting of the article was performed by M. Baudat. The final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baudat, M., Joosten, E.A.J. & Simons, S.H.P. Repetitive daily oxytocin treatment reduces weight gain but not acute neonatal procedural pain. Pediatr Res 98, 294–300 (2025). https://doi.org/10.1038/s41390-024-03680-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03680-9