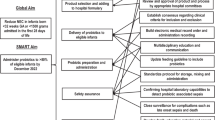
Abstract
Background
Probiotic administration may decrease the incidence of necrotizing enterocolitis (NEC) through mechanisms that are largely unknown. We investigated the effects of probiotics on intestinal epigenetics and assessed their effects on intestinal inflammation and motility using both ileum-predominant and combined ileo-colitis mouse NEC models.
Methods
C57BL/6 J mice were gavage-fed a multi-strain probiotic from postnatal days 3-11, consisting of B. infantis, B. lactis, and S. thermophilus. From p8, mice were exposed to ileo-colitis NEC involving formula containing NEC bacteria and 0.5% DSS. DNA methylation was measured using the Infinium Methylation Assay. Gastrointestinal motility was assessed by 70 Kd FITC-dextran transit time. Probiotic colonization was measured in probiotic-fed mice by qPCR.
Results
Probiotic administration caused significant changes in the small intestine’s epigenetic signature, a reduction in NEC severity, and improved intestinal motility. The effects of probiotics were more pronounced in the ileo-colitis NEC model.
Conclusions
These findings shed light on the role of probiotics in two clinically relevant models of NEC, add additional insights into their underlying mechanism of action, and reveal unanticipated epigenetic modifications to the intestinal mucosa after their use.
Impact
-
These findings shed light on the role of multi-strain probiotics in two clinically relevant animal models of NEC, and add additional insights into their underlying mechanism of action
-
This study provides a new, clinically relevant model for the study of NEC including administration of 0.5% DSS, to include ileal dominant and ileo-colonic dominant phenotypes of the disease.
-
These results reveal that clinically relevant strains of probiotic bacteria can exert epigenetic effects on the small intestine in mice, and can attenuate the epigenetic changes induced by NEC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are partially available in the supplementary material, additional information is available from the corresponding author on reasonable request. The R-scripts used for DNA methylation analyses are available on Github https://github.com/hannahmoore6/Multi-strain-Probiotic-Administration-Methylation-Assay.
References
Hackam, D. J. & Sodhi, C. P. Bench to bedside—new insights into the pathogenesis of necrotizing enterocolitis. Nat. Rev. Gastroenterol. Hepatol. 19, 468–479 (2022).
Lin, P. W. & Stoll, B. J. Necrotising enterocolitis. Lancet 368, 1271–1283 (2006).
Pammi, M. et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5, 31 (2017).
Scheese, D. J., Sodhi, C. P. & Hackam, D. J. New insights into the pathogenesis of necrotizing enterocolitis and the dawn of potential therapeutics. Semin Pediatr. Surg. 32, 151309 (2023).
Singh, D. K. et al. Necrotizing enterocolitis: bench to bedside approaches and advancing our understanding of disease pathogenesis. Front Pediatr. 10, 1107404 (2022).
Cilieborg, M. S., Boye, M. & Sangild, P. T. Bacterial colonization and gut development in preterm neonates. Early Hum. Dev. 88, S41–S49 (2012).
Yu, D. H. et al. Postnatal epigenetic regulation of intestinal stem cells requires DNA methylation and is guided by the microbiome. Genome Biol. 16, 211 (2015).
Good, M. et al. Global hypermethylation of intestinal epithelial cells is a Hallmark feature of neonatal surgical necrotizing enterocolitis. Clin. Epigenet. 12, 190 (2020).
Good, M. et al. Neonatal necrotizing enterocolitis-associated DNA methylation signatures in the colon are evident in stool samples of affected individuals. Epigenomics 13, 829–844 (2021).
Tian, B. et al. Epigenetic insights into necrotizing enterocolitis: unraveling methylation-regulated biomarkers. Inflammation (2024).
Liu, Y., Fatheree, N. Y., Mangalat, N. & Rhoads, J. M. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of Tlr4 and Nf-Kappab signaling in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G608–G617 (2012).
Jilling, T., Lu, J., Jackson, M. & Caplan, M. S. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr. Res. 55, 622–629 (2004).
Afrazi, A. et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J. Biol. Chem. 289, 9584–9599 (2014).
Werts, A. D. et al. A novel role for necroptosis in the pathogenesis of necrotizing enterocolitis. Cell Mol. Gastroenterol. Hepatol. 9, 403–423 (2020).
Sodhi, C. P. et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 138, 185–196 (2010).
Neal, M. D. et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the P53 up-regulated modulator of apoptosis. J. Biol. Chem. 287, 37296–37308 (2012).
Sodhi, C. P. et al. The human milk oligosaccharides 2’-fucosyllactose and 6’-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr. Res. 89, 91–101 (2021).
Good, M. et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 8, 1166–1179 (2015).
Sodhi, C. P. et al. Intestinal epithelial toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143, 708–718 e705 (2012).
Egan, C. E. et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J. Clin. Invest 126, 495–508 (2016).
Gribar, S. C. et al. Reciprocal expression and signaling of Tlr4 and Tlr9 in the pathogenesis and treatment of necrotizing enterocolitis. J. Immunol. 182, 636–646 (2009).
Tran, L. et al. Necrotizing enterocolitis and cytomegalovirus infection in a premature infant. Pediatrics 131, e318–e322 (2013).
Hackam, D. J., Good, M. & Sodhi, C. P. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: toll-like receptors throw the switch. Semin Pediatr. Surg. 22, 76–82 (2013).
Kashif, H., Abuelgasim, E., Hussain, N., Luyt, J. & Harky, A. Necrotizing enterocolitis and congenital heart disease. Ann. Pediatr. Cardiol. 14, 507–515 (2021).
Garg, P. M. et al. Intestinal resection is more likely to be effective in necrotizing enterocolitis extending to colon than in disease limited to the small intestine. Newborn (Clarksville) 1, 14–26 (2022).
Wang, Y. et al. Probiotics, prebiotics, lactoferrin, and combination products for prevention of mortality and morbidity in preterm infants: a systematic review and network meta-analysis. JAMA Pediatr. 177, 1158–1167 (2023).
Hill, C. et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014).
Chi, C. et al. Effects of probiotics in preterm infants: a network meta-analysis. Pediatrics 147, e20200706 (2021).
Sharif, S., Meader, N., Oddie, S. J., Rojas-Reyes, M. X. & McGuire, W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst. Rev. 7, CD005496 (2023).
Barbian, M. E. & Patel, R. M. Probiotics for prevention of necrotizing enterocolitis: where do we stand? Semin. Perinatol. 47, 151689 (2023).
Underwood, M. A. et al. Bifidobacterium longum Subsp. Infantis in experimental necrotizing enterocolitis: alterations in inflammation, innate immune response, and the microbiota. Pediatr. Res. 76, 326–333 (2014).
Shiou, S. R. et al. Synergistic protection of combined probiotic conditioned media against neonatal necrotizing enterocolitis-like intestinal injury. PLoS One 8, e65108 (2013).
Alsharairi, N. A. Therapeutic potential of gut microbiota and its metabolite short-chain fatty acids in neonatal necrotizing enterocolitis. Life (Basel) 13, 561 (2023).
Sadeghpour Heravi, F. & Hu, H. Bifidobacterium: host-microbiome interaction and mechanism of action in preventing common gut-microbiota-associated complications in preterm infants: a narrative review. Nutrients 15, 709 (2023).
Patel, R. M. & Underwood, M. A. Probiotics and necrotizing enterocolitis. Semin. Pediatr. Surg. 27, 39–46 (2018).
Luo, Z. et al. Limosilactobacillus reuteri in immunomodulation: molecular mechanisms and potential applications. Front Immunol. 14, 1228754 (2023).
Good, M. et al. Lactobacillus rhamnosus Hn001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of Tlr9. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G1021–G1032 (2014).
Ansari, I. et al. The microbiota programs DNA methylation to control intestinal homeostasis and inflammation. Nat. Microbiol. 5, 610–619 (2020).
Cortese, R., Lu, L., Yu, Y., Ruden, D. & Claud, E. C. Epigenome-microbiome crosstalk: a potential new paradigm influencing neonatal susceptibility to disease. Epigenetics 11, 205–215 (2016).
Plummer, E. L. et al. Gut microbiota of preterm infants supplemented with probiotics: sub-study of the proprems trial. BMC Microbiol. 18, 184 (2018).
Zhang, W. et al. Clinical efficacy of probiotics on feeding intolerance in preterm infants: a systematic review and meta-analysis. Transl. Pediatr. 11, 229–238 (2022).
Kovler, M. L. et al. Toll-like receptor 4-mediated enteric glia loss is critical for the development of necrotizing enterocolitis. Sci. Transl. Med. 13, eabg3459 (2021).
van den Akker, C. H. P. et al. Probiotics and preterm infants: a position paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 70, 664–680 (2020).
Jacobs, S. E. et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 132, 1055–1062 (2013).
Miller, M. S., Galligan, J. J. & Burks, T. F. Accurate measurement of intestinal transit in the rat. J. Pharm. Methods 6, 211–217 (1981).
Zhou, W. et al. DNA methylation dynamics and dysregulation delineated by high-throughput profiling in the mouse. Cell Genom. 2, 100144 (2022).
Zhou, W., Triche, T. J. Jr., Laird, P. W. & Shen, H. Sesame: reducing artifactual detection of DNA methylation by infinium beadchips in genomic deletions. Nucleic Acids Res. 46, e123 (2018).
Triche, T. J. Jr., Weisenberger, D. J., Van Den Berg, D., Laird, P. W. & Siegmund, K. D. Low-level processing of illumina infinium DNA methylation beadarrays. Nucleic Acids Res. 41, e90 (2013).
Summarizedexperiment: Summarizedexperiment Container. R Package Version 1.32.0, https://Bioconductor.Org/Packages/Summarizedexperiment (2023).
Viennois, E., Chen, F., Laroui, H., Baker, M. T. & Merlin, D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res. Notes 6, 360 (2013).
Sodhi, C. P. et al. Fat composition in infant formula contributes to the severity of necrotising enterocolitis. Br. J. Nutr. 120, 665–680 (2018).
Quaroni, A., Isselbacher, K. J. & Ruoslahti, E. Fibronectin synthesis by epithelial crypt cells of rat small intestine. Proc. Natl. Acad. Sci. USA 75, 5548–5552 (1978).
Good, M. et al. Amniotic fluid inhibits toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc. Natl. Acad. Sci. USA 109, 11330–11335 (2012).
Remon, J. I. et al. Depth of bacterial invasion in resected intestinal tissue predicts mortality in surgical necrotizing enterocolitis. J. Perinatol. 35, 755–762 (2015).
Lambert, D. K. et al. Necrotizing enterocolitis in term neonates: data from a multihospital health-care system. J. Perinatol. 27, 437–443 (2007).
Chassaing, B., Aitken, J. D., Malleshappa, M. & Vijay-Kumar, M. Dextran sulfate sodium (Dss)-induced colitis in mice. Curr. Protoc. Immunol. 104, 15 25 11–15 25 14 (2014).
Ginzel, M. et al. Dextran sodium sulfate (Dss) induces necrotizing enterocolitis-like lesions in neonatal mice. PLoS One 12, e0182732 (2017).
Leaphart, C. L. et al. A critical role for Tlr4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J. Immunol. 179, 4808–4820 (2007).
Cao, M. et al. Physical activity and gastric residuals as biomarkers for region-specific nec lesions in preterm neonates. Neonatology 110, 241–247 (2016).
Pan, X. et al. Blood transcriptomic markers of necrotizing enterocolitis in preterm pigs. Pediatr. Res. 91, 1113–1120 (2022).
Raouf, Z. et al. Colitis-induced small intestinal hypomotility is dependent on enteroendocrine cell loss in mice. Cell Mol. Gastroenterol. Hepatol. 18, 53–70 (2024).
Henrick, B. M. et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898 e3811 (2021).
Toumi, R. et al. Beneficial role of the probiotic mixture ultrabiotique on maintaining the integrity of intestinal mucosal barrier in Dss-induced experimental colitis. Immunopharmacol. Immunotoxicol. 35, 403–409 (2013).
Zhang, Y. et al. Probiotic mixture protects dextran sulfate sodium-induced colitis by altering tight junction protein expressions and increasing Tregs. Med. Inflamm. 2018, 9416391 (2018).
Khailova, L. et al. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G940–G949 (2009).
Yan, F. & Polk, D. B. Probiotics and probiotic-derived functional factors-mechanistic insights into applications for intestinal homeostasis. Front Immunol. 11, 1428 (2020).
Walsh, C., Lane, J. A., Van Sinderen, D. & Hickey, R. M. Human milk oligosaccharide-sharing by a consortium of infant derived bifidobacterium species. Sci. Rep. 12, 4143 (2022).
Chandrasekharan, B. et al. Interactions between commensal bacteria and enteric neurons, via Fpr1 induction of ros, increase gastrointestinal motility in mice. Gastroenterology 157, 179–192 e172 (2019).
Dimidi, E., Christodoulides, S., Fragkos, K. C., Scott, S. M. & Whelan, K. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 100, 1075–1084 (2014).
Indrio, F. et al. The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J. Pediatr. 152, 801–806 (2008).
Chen, W. et al. Gut transit time, using radiological contrast imaging, to predict early signs of necrotizing enterocolitis. Pediatr. Res. 89, 127–133 (2021).
Mischke, M. & Plosch, T. The gut microbiota and their metabolites: potential implications for the host epigenome. Adv. Exp. Med. Biol. 902, 33–44 (2016).
Merid, S. K. et al. Epigenome-wide meta-analysis of blood DNA methylation in newborns and children identifies numerous loci related to gestational age. Genome Med. 12, 25 (2020).
Al-Hadidi, A., Navarro, J., Goodman, S. D., Bailey, M. T. & Besner, G. E. Lactobacillus reuteri in its biofilm state improves protection from experimental necrotizing enterocolitis. Nutrients 13, 918 (2021).
Acknowledgements
We would like to thank all the undergrad students involved in this research project for their help, in particular Thomas Cancian, Diego Kaune and Dylan Yoon, as well as the supporting staff of the Johns Hopkins Miller Research Building Animal Facility, particularly Nancy Lim. We would also like to thank Roxann Ashworth, Co-Director of the Johns Hopkins Genetic Resources Core Facility (GRCF) for her assistance with the DNA methylation analyses. All schematic model overviews in the figures were created using Biorender.com.
Funding
D.H.K. was supported by a Royal Netherlands Academy of Arts and Sciences (KNAW) Ter Meulen Grant. D.J.H. is supported by R35 GM141956 and C.T., C.L. and D.S. are supported by T32DK007713.
Author information
Authors and Affiliations
Contributions
D.J.H., C.P.S., D.H.K., H.M., D.J.S., C.T., Z.R., J.W.D., K.T., M.E.S., S.W., M.G.E.B., H.J., T.P., S.W., M.W., and W.B.F. made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. D.J.H., C.P.S., D.H.K. and H.M. drafted the article or revised it critically for important intellectual content; and all authors gave their final approval of the version to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Klerk, D.H., Moore, H., Scheese, D.J. et al. Multi-strain probiotic administration decreases necrotizing enterocolitis severity and alters the epigenetic profile in mice. Pediatr Res 98, 1559–1569 (2025). https://doi.org/10.1038/s41390-024-03716-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03716-0
This article is cited by
-
Biofilm probiotics for NEC: promise, pitfalls, and pathways to translation
Pediatric Research (2025)
-
Epigenetic changes associated with severe necrosis and survival in preterm infants with surgical necrotizing enterocolitis
Pediatric Research (2025)