Abstract
Objective
This study investigates the causal relationship between gut microbiota, inflammatory cytokines, and Kawasaki disease (KD), and whether cytokines mediate the effect of gut microbiota on KD.
Methods
A Mendelian randomization analysis using the inverse-variance weighted method assessed the causal effects of gut microbiota and inflammatory cytokines on KD and explored potential mediation.
Results
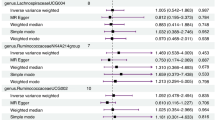
The study found causal links between 20 types of gut microbiota and KD. Ten types increased KD risk, notably Francisellales (OR = 27.82, P = 0.0309). Ten types provided protection, with Fusobacteriaceae showing the strongest effect (OR = 0.0424, P = 0.002). Five inflammatory cytokines were significantly associated with KD; adenosine deaminase was most protective (OR = 0.7447, P = 0.0037), while Fractalkine indicated higher risk (OR = 2.0448, P = 0.0315). Mediation analysis revealed that the Interleukin-10 receptor subunit beta mediates the effect of Bifidobacterium adolescentis on KD, with a mediation effect of −0.0237 (4.75% ratio). Interleukin-20 mediates the effect of Faecalicatena lactaris on KD, with a mediation effect of −0.1168 (15.30% ratio).
Conclusion
The findings indicate a causal relationship among gut microbiota, inflammatory cytokines, and KD, suggesting that the gut microbiome influences KD through specific cytokines.
Impact
-
The study confirmed a causal relationship between 20 types of gut microbiota and Kawasaki disease, finding that 10 types increase the risk of Kawasaki disease, particularly Francisellales.
-
Five inflammatory cytokines were significantly associated with Kawasaki disease, with adenosine deaminase showing a protective effect, while Fractalkine increased the risk.
-
Mediation analysis indicated that specific inflammatory cytokines (such as Interleukin-10 receptor subunit beta and Interleukin-20) play a significant mediating role between gut microbiota and Kawasaki disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available in the Finngen database (https://www.finngen.fi/en) and IEU OpenGWAS (https://gwas.mrcieu.ac.uk/).
References
Burns, J. C. The etiologies of Kawasaki disease. J. Clin. Investig. 134, e176938 (2024).
Selmek, K. & Harding, M. Kawasaki disease. Pediatr. Rev. 45, 425–427 (2024).
Day-Lewis, M. et al. Kawasaki disease: contemporary perspectives. Lancet Child Adolesc. Health 8, 781–792 (2024).
Xu, L. et al. A bibliometric analysis of Kawasaki disease from 1974 to 2022. Heliyon 10, e27290 (2024).
Teramoto, Y. et al. Dysbiosis of the gut microbiota as a susceptibility factor for Kawasaki disease. Front. Immunol. 14, 1268453 (2023).
Brodeur, K. E. et al. Elevation of IL-17 cytokines distinguishes Kawasaki disease from other pediatric inflammatory disorders. Arthritis Rheumatol. 76, 285–292 (2024).
Gomaa, E. Z. Human gut microbiota/microbiome in health and diseases: a review. Antonie Leeuwenhoek 113, 2019–2040 (2020).
Chong, W. P. Editorial for the special issue “Cytokines in Inflammatory Signaling”. Int. J. Mol. Sci. 25, 5799 (2024).
Burgess, S. et al. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat. Med. 35, 1880–1906 (2016).
Bowden, J. & Holmes, M. V. Meta-analysis and Mendelian randomization: a review. Res. Synth. Methods 10, 486–496 (2019).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA 326, 1614–1621 (2021).
Qin, Y. et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat. Genet. 54, 134–142 (2022).
Zhao, J. H. et al. Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat. Immunol. 24, 1540–1551 (2023).
Buda, P. et al. Association between rs12037447, rs146732504, rs151078858, rs55723436, and rs6094136 polymorphisms and Kawasaki disease in the population of Polish children. Front. Pediatr. 9, 624798 (2021).
Ganapathiraju, M. K. et al. A reference catalog of DNA palindromes in the human genome and their variations in 1000 Genomes. Hum. Genome Var. 7, 40 (2020).
Burgess, S. et al. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 26, 2333–2355 (2017).
Slob, E. & Burgess, S. A comparison of robust Mendelian randomization methods using summary data. Genet. Epidemiol. 44, 313–329 (2020).
Bowden, J. et al. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Belkaid, Y. & Harrison, O. J. Homeostatic immunity and the microbiota. Immunity 46, 562–576 (2017).
Dinakis, E. et al. The gut-immune axis during hypertension and cardiovascular diseases. Acta Physiol. 240, e14193 (2024).
Kwack, D. W. et al. Changes in gut microbiome can be associated with abrupt seizure exacerbation in epilepsy patients. Clin. Neurol. Neurosurg. 246, 108556 (2024).
Filidou, E. et al. Probiotics: shaping the gut immunological responses. World J. Gastroenterol. 30, 2096–2108 (2024).
Gavzy, S. J. et al. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 15, 2291164 (2023).
Gao, Z. W. et al. The roles of adenosine deaminase in autoimmune diseases. Autoimmun. Rev. 20, 102709 (2021).
Rodriguez, C. et al. Fractalkine in health and disease. Int. J. Mol. Sci. 25, 8007 (2024).
Haybar, H. et al. Cytokines and their role in cardiovascular diseases. Cytokine 169, 156261 (2023).
Zhou, B. et al. Intestinal flora and disease mutually shape the regional immune system in the intestinal tract. Front. Immunol. 11, 575 (2020).
Hays, K. E. et al. The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes 16, 2393270 (2024).
Acknowledgements
This study was funded by the Guangxi Science and Technology Program Project (Guike AD22035121).
Author information
Authors and Affiliations
Contributions
J.G.W.: full access to all data and responsibility for the integrity of the data and the accuracy of the analysis, study concept and design, acquisition, analysis, and interpretation of data, and drafting of the manuscript; J.G.W. and H.H.D.: analysis and interpretation of data, drafting of the manuscript. Q.Y.L.: acquisition, analysis, and interpretation of data, and funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
In this MR study, we used publicly available aggregate data; therefore, no separate ethical approval is required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, JG., Dou, HH. & Liang, QY. Gut microbiota, inflammatory cytokines, and Kawasaki disease: a Mendelian randomization study and mediation analysis. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-03911-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-03911-7