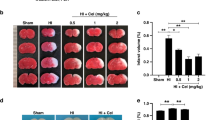
Abstract
Background
Hypoxic ischemia (HI) is one of the common causes of neonatal brain injury, leading to neurodevelopmental disorders such as cerebral palsy, epilepsy, and cognitive deficits. In this study, we evaluated neuroprotective effects of vitamin C on the neonatal HI brain injury mouse model.
Methods
Brain damage measurement, sensorimotor function in the neonatal period, learning and memory ability in adulthood were carried out in mice treated with vitamin C daily for 7 consecutive days following HI brain injury at postnatal day 7.
Results
Vitamin C treatment significantly reduced the hippocampus damage area, infarction volume, hippocampal neuron loss, and suppressed the neuroinflammation after HI injury. Additionally, it improved performance on neonatal sensorimotor function tests and learning and memory ability in adulthood.
Conclusions
Vitamin C reduced brain injury and improved functional recovery in the neonatal hypoxic ischemia brain damage (HIBD) model.
Impact
-
Vitamin C treatment significantly reduced neuron loss and suppressed the neuroinflammation after hypoxic-ischemic brain injury.
-
Vitamin C treatment enhanced sensorimotor functions in neonates and improved cognitive abilities in adults after hypoxic-ischemic brain injury.
-
Vitamin C could be an attractive candidate drug in clinical trials of hypoxic-ischemic encephalopathy therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Douglas-Escobar, M. & Weiss, M. D. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 169, 397–403 (2015).
Lee, B. L. & Glass, H. C. Cognitive outcomes in late childhood and adolescence of neonatal hypoxic-ischemic encephalopathy. Clin. Exp. Pediatr. 64, 608–618 (2021).
Jacobs, S., Hunt, R., Tarnow-Mordi, W., Inder, T. & Davis, P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, Cd003311 (2007).
Parikh, P. & Juul, S. E. Neuroprotective strategies in neonatal brain injury. J. Pediatr. 192, 22–32 (2018).
Zheng, H., Xu, Y., Liehn, E. A. & Rusu, M. Vitamin C as scavenger of reactive oxygen species during healing after myocardial infarction. Int. J. Mol. Sci. 25, 3114(2024).
Hansen, S. N., Tveden-Nyborg, P. & Lykkesfeldt, J. Does vitamin C deficiency affect cognitive development and function? Nutrients 6, 3818–3846 (2014).
Flashman, E., Davies, S. L., Yeoh, K. K. & Schofield, C. J. Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting hif and prolyl hydroxylase domain 2) on ascorbate and other reducing agents. Biochem. J. 427, 135–142 (2010).
Tveden-Nyborg, P. et al. Vitamin C deficiency in early postnatal life impairs spatial memory and reduces the number of hippocampal neurons in guinea pigs. Am. J. Clin. Nutr. 90, 540–546 (2009).
Rice, M. E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 23, 209–216 (2000).
Tveden-Nyborg, P. & Lykkesfeldt, J. Does vitamin C deficiency increase lifestyle-associated vascular disease progression? Evidence based on experimental and clinical studies. Antioxid. Redox Signal 19, 2084–2104 (2013).
Bowman, G. L. Ascorbic acid, cognitive function, and Alzheimer’s disease: a current review and future direction. Biofactors 38, 114–122 (2012).
Harrison, F. E. & May, J. M. Vitamin C function in the brain: vital role of the ascorbate transporter Svct2. Free Radic. Biol. Med. 46, 719–730 (2009).
Moretti, M., Fraga, D. B. & Rodrigues, A. L. S. Ascorbic acid to manage psychiatric disorders. CNS Drugs 31, 571–583 (2017).
Shah, S. A., Yoon, G. H., Kim, H. O. & Kim, M. O. Vitamin C neuroprotection against dose-dependent glutamate-induced neurodegeneration in the postnatal brain. Neurochem. Res. 40, 875–884 (2015).
Levine, M. et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc. Natl Acad. Sci. USA 93, 3704–3709 (1996).
Lykkesfeldt, J. & Poulsen, H. E. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br. J. Nutr. 103, 1251–1259 (2010).
Yanase, F. et al. Harm of IV high-dose vitamin C therapy in adult patients: a scoping review. Crit. Care Med. 48, e620–e628 (2020).
Vega Franco, L. Development of the digestive system. medico-dietetic implications in infants. Rev. Investig. Clin. 45, 605–612 (1993).
Indrio, F. et al. Development of the gastrointestinal tract in newborns as a challenge for an appropriate nutrition: a narrative review. Nutrients 14, 1405 (2022).
Smith, A. L., Alexander, M., Rosenkrantz, T. S., Sadek, M. L. & Fitch, R. H. Sex differences in behavioral outcome following neonatal hypoxia ischemia: insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic brain injury. Exp. Neurol. 254, 54–67 (2014).
Albertsson, A. M. et al. The immune response after hypoxia-ischemia in a mouse model of preterm brain injury. J. Neuroinflam. 11, 153 (2014).
Ji, X. et al. Functional reconstruction of the basal ganglia neural circuit by human striatal neurons in hypoxic-ischaemic injured brain. Brain 146, 612–628 (2023).
Fischer, A. H., Jacobson, K. A., Rose, J. & Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, pdb.prot4986 (2008).
Zhang, Q. et al. Tlr3 deletion inhibits programmed necrosis of brain cells in neonatal mice with sevoflurane-induced cognitive dysfunction. Aging 14, 4714–4727 (2022).
Sampath, D. et al. Effects of a potassium channel opener on brain injury and neurologic outcomes in an animal model of neonatal hypoxic-ischemic injury. Pediatr. Res. 88, 202–208 (2020).
Feather-Schussler, D. N. & Ferguson, T. S. A battery of motor tests in a neonatal mouse model of cerebral Palsy. J. Vis. Exp. 53569 (2016).
Yokoi, F., Dang, M. T., Zhou, T. & Li, Y. Abnormal nuclear envelopes in the striatum and motor deficits in Dyt11 myoclonus-dystonia mouse models. Hum. Mol. Genet. 21, 916–925 (2012).
Jiang, P. et al. HESC-derived olig2+ progenitors generate a subtype of astroglia with protective effects against ischaemic brain injury. Nat. Commun. 4, 2196 (2013).
Kocot, J., Luchowska-Kocot, D., Kiełczykowska, M., Musik, I. & Kurzepa, J. Does vitamin C influence neurodegenerative diseases and psychiatric disorders? Nutrients 9, 659(2017).
Yan, M. et al. High-dose ascorbic acid administration improves functional recovery in rats with spinal cord contusion injury. Spinal Cord. 52, 803–808 (2014).
Morris-Blanco, K. C. et al. High-dose vitamin C prevents secondary brain damage after stroke via epigenetic reprogramming of neuroprotective genes. Transl. Stroke Res. 13, 1017–1036 (2022).
Spoelstra-de Man, A. M. E., Elbers, P. W. G. & Oudemans-van Straaten, H. M. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit. Care 22, 70 (2018).
Yuan, J., Lipinski, M. & Degterev, A. Diversity in the mechanisms of neuronal cell death. Neuron 40, 401–413 (2003).
Gonçalves, J. T., Schafer, S. T. & Gage, F. H. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell 167, 897–914 (2016).
Rehman, S. U., Shah, S. A., Ali, T., Chung, J. I. & Kim, M. O. Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol. Neurobiol. 54, 255–271 (2017).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Tang, Y. & Le, W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 53, 1181–1194 (2016).
Modrell, A. K. & Tadi, P. in StatPearls (StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC., 2024).
Chen, J. et al. A Myt1l syndrome mouse model recapitulates patient phenotypes and reveals altered brain development due to disrupted neuronal maturation. Neuron 109, 3775–3792.e3714 (2021).
Campos, F. L. et al. Rodent models of Parkinson’s disease: beyond the motor symptomatology. Front. Behav. Neurosci. 7, 175 (2013).
Vorhees, C. V. & Williams, M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858 (2006).
Lőrincz, T., Holczer, M., Kapuy, O. & Szarka, A. The interrelationship of pharmacologic ascorbate induced cell death and ferroptosis. Pathol. Oncol. Res 25, 669–679 (2019).
de Sales, K. P. F. et al. Effects of vitamin C on the prevention of ischemia-reperfusion brain injury: experimental study in rats. Int J. Vasc. Med. 2019, 4090549 (2019).
Chang, C. Y., Chen, J. Y., Wu, M. H. & Hu, M. L. Therapeutic treatment with vitamin C reduces focal cerebral ischemia-induced brain infarction in rats by attenuating disruptions of blood brain barrier and cerebral neuronal apoptosis. Free Radic. Biol. Med. 155, 29–36 (2020).
Lin, J. L., Huang, Y. H., Shen, Y. C., Huang, H. C. & Liu, P. H. Ascorbic acid prevents blood-brain barrier disruption and sensory deficit caused by sustained compression of primary somatosensory cortex. J. Cereb. Blood Flow. Metab. 30, 1121–1136 (2010).
Hong, J. Y., Davaa, G., Yoo, H., Hong, K. & Hyun, J. K. Ascorbic acid promotes functional restoration after spinal cord injury partly by epigenetic modulation. Cells 9, 1310 (2020).
Reagan-Shaw, S., Nihal, M. & Ahmad, N. Dose translation from animal to human studies revisited. Faseb J. 22, 659–661 (2008).
Bass, W. T., Malati, N., Castle, M. C. & White, L. E. Evidence for the safety of ascorbic acid administration to the premature infant. Am. J. Perinatol. 15, 133–140 (1998).
Farooqi, I. S. & Xu, Y. Translational potential of mouse models of human metabolic disease. Cell 187, 4129–4143 (2024).
Lembo, C., Buonocore, G. & Perrone, S. Oxidative stress in preterm newborns. Antioxidants 10, 1672 (2021).
Nakajima, A., Ueda, Y., Sameshima, H. & Ikenoue, T. Intracerebral antioxidant ability of mature rats after neonatal hypoxic-ischemic brain injury estimated using the microdialysis-electron spin resonance method. J. Obstet. Gynaecol. Res. 41, 884–889 (2015).
Montaldo, P., Pauliah, S. S., Lally, P. J., Olson, L. & Thayyil, S. Cooling in a low-resource environment: lost in translation. Semin Fetal Neonatal Med. 20, 72–79 (2015).
Gunn, A. J. et al. Therapeutic hypothermia translates from ancient history in to practice. Pediatr. Res. 81, 202–209 (2017).
Favrais, G. et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 70, 550–565 (2011).
Leviton, A. & Gressens, P. Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 30, 473–478 (2007).
Tóth, S. Z., Lőrincz, T. & Szarka, A. Concentration does matter: the beneficial and potentially harmful effects of ascorbate in humans and plants. Antioxid. Redox Signal 29, 1516–1533 (2018).
Liu, D., Pei, D., Hu, H., Gu, G. & Cui, W. Effects and mechanisms of vitamin C post-conditioning on platelet activation after hypoxia/reoxygenation. Transfus. Med. Hemother. 47, 110–118 (2020).
Guaiquil, V. H., Golde, D. W., Beckles, D. L., Mascareno, E. J. & Siddiqui, M. A. Vitamin C inhibits hypoxia-induced damage and apoptotic signaling pathways in cardiomyocytes and ischemic hearts. Free Radic. Biol. Med 37, 1419–1429 (2004).
Kaźmierczak-Barańska, J., Boguszewska, K., Adamus-Grabicka, A. & Karwowski, B. T. Two faces of vitamin C-antioxidative and pro-oxidative agent. Nutrients 12, 1501 (2020).
Kawashima, A. et al. Vitamin C induces the reduction of oxidative stress and paradoxically stimulates the apoptotic gene expression in extravillous trophoblasts derived from first-trimester tissue. Reprod. Sci. 22, 783–790 (2015).
Di Tano, M. et al. Synergistic effect of fasting-mimicking diet and vitamin C against Kras mutated cancers. Nat. Commun. 11, 2332 (2020).
Zhou, J. et al. Vitamin C promotes apoptosis and cell cycle arrest in oral squamous cell carcinoma. Front. Oncol. 10, 976 (2020).
Abbah, J. et al. Oxidative stress-induced damage to the developing hippocampus is mediated by Gsk3β. J. Neurosci. 42, 4812–4827 (2022).
Cutuli, D. et al. Behavioral, neuromorphological, and neurobiochemical effects induced by omega-3 fatty acids following basal forebrain cholinergic depletion in aged mice. Alzheimers Res. Ther. 12, 150 (2020).
Sahay, A. & Hen, R. Hippocampal neurogenesis and depression. Novartis Found. Symp. 289, 152–160 (2008).
O’Donnell, P. & Grace, A. A. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J. Neurosci. 15, 3622–3639 (1995).
Pikkarainen, M., Rönkkö, S., Savander, V., Insausti, R. & Pitkänen, A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J. Comp. Neurol. 403, 229–260 (1999).
Bird, C. M. & Burgess, N. The hippocampus and memory: insights from spatial processing. Nat. Rev. Neurosci. 9, 182–194 (2008).
Rao, Y. L. et al. Hippocampus and its involvement in Alzheimer’s disease: a review. 3 Biotech. 12, 55 (2022).
Gray, J. A. A theory of anxiety: the role of the limbic system. Encephale 9, 161b–166b (1983).
Kheirbek, M. A. et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77, 955–968 (2013).
Jimenez, J. C. et al. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97, 670–683.e676 (2018).
Padilla-Coreano, N. et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron 89, 857–866 (2016).
Mirza, M. A., Ritzel, R., Xu, Y., McCullough, L. D. & Liu, F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J. Neuroinflam. 12, 32 (2015).
Kelly, L. A., Branagan, A., Semova, G. & Molloy, E. J. Sex differences in neonatal brain injury and inflammation. Front. Immunol. 14, 1243364 (2023).
Hayes, B. C. et al. A case-control study of hypoxic-ischemic encephalopathy in newborn infants at >36 weeks gestation. Am. J. Obstet. Gynecol. 209, 29.e21–29.e19 (2013).
Chalak, L. F., Pruszynski, J. E. & Spong, C. Y. Sex vulnerabilities to hypoxia-ischemia at birth. JAMA Netw. Open 6, e2326542 (2023).
Acknowledgements
This work was supported by the fund for Less Developed Regions of the National Natural Science Foundation of China (No. 82260316) to B.Z.
Author information
Authors and Affiliations
Contributions
Kaiyi Liu and Xiaoqing Chen contributed equally to this work. Yifeng Lin and Wenhao Zhou designed the study and supervised the experiments; Kaiyi Liu and Xiaoqing Chen carried out most of the experiments, analyzed results, and wrote the manuscript; Fangbing Chen, Wenjuan Dai, Shiyi Zheng and Bi Ze helped with some experiments. All authors revised the original manuscript and agreed on its contents.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, K., Chen, X., Chen, F. et al. Neuroprotective effects of vitamin C on hypoxic-ischemic brain injury in neonatal mice. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-03926-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-03926-0