Abstract
Background
The relationship between early antibiotic exposure, necrotizing enterocolitis (NEC), and growth faltering (GF) in extremely preterm infants is unknown.
Methods
We evaluated the association between peripartum and postnatal antibiotic exposure in the first week after birth with NEC and GF in this secondary analysis of Preterm Erythropoietin Neuroprotection Trial subjects. NEC was defined as Bell’s stage ≥ IIA; GF was defined as decreased weight, length, or head circumference (HC) z-score from birth to discharge of < −0.8. Multivariable analyses were adjusted with maternal and infant factors.
Results
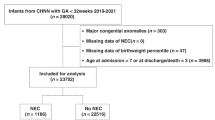
A total of 891 infants survived the first week and were included in the NEC analyses, while 828 infants survived to discharge and were included in the growth analyses. For every 1-day increase in infant antibiotic exposure during the first week after birth, there was a significantly increased adjusted hazard of NEC (aHR/day 1.14 [1.01–1.28], p = 0.034). Antibiotics for 3–4 days and 5–7 days total in the first week were associated with increased odds of weight GF (aOR 1.90 [1.21–2.99], aOR 2.32 [1.44–3.74]), length GF (aOR 1.76 [1.22–2.59], aOR 1.88 [1.26–2.80]), and HC GF (aOR 1.75 [1.08–2.84], aOR 1.87 [1.14–3.08]).
Conclusion
Increased antibiotic exposure in the first week after birth was associated with NEC and GF risk.
Impact
-
In this post-hoc analysis of a large multi-site trial, we found infant antibiotic exposure in the first week after birth was associated with an increased hazard of necrotizing enterocolitis in the extremely preterm infant after adjusting for maternal and infant factors.
-
First week antibiotic exposure in the extremely preterm infant was associated with an increased odds of weight, linear, and head circumference growth faltering after adjusting for maternal and infant factors.
-
These findings encourage the judicious use of early antibiotics in extremely preterm infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
De-identified individual participant data are available through the NINDS Data Archive under “Clinical Research Datasets.” The data are de-identified and a limited access data set is available through a request form on that page. Data dictionaries, in addition to the study protocol, the statistical analysis plan, and the informed consent form will be included. The data will be made available upon publication of all PENUT Trial-related manuscripts to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal.
References
Kostlin-Gille, N. et al. Early initiation of antibiotic therapy and short-term outcomes in preterm infants: a single-centre retrospective cohort analysis. Arch. Dis. Child Fetal Neonatal Ed. 108, 623–630 (2023).
Letouzey, M. et al. Early antibiotic exposure and adverse outcomes in very preterm infants at low risk of early-onset sepsis: the Epipage-2 cohort study. J. Pediatr. 243, 91–98.e94 (2022).
Dierikx, T. H. et al. Association between duration of early empiric antibiotics and necrotizing enterocolitis and late-onset sepsis in preterm infants: a multicenter cohort study. Eur. J. Pediatr. 181, 3715–3724 (2022).
Vatne, A. et al. Early empirical antibiotics and adverse clinical outcomes in infants born very preterm: a population-based cohort. J. Pediatr. 253, 107–114.e105 (2023).
Perez, K. et al. Patterns of infections among extremely preterm infants. J. Clin. Med. 12, 2703 (2023).
Flannery, D. D., Edwards, E. M., Puopolo, K. M. & Horbar, J. D. Early-onset sepsis among very preterm infants. Pediatrics 148, e2021052456 (2021).
Lynch, T. A., Malshe, A., Dozier, A. & Seplaki, C. L. Preterm prelabor rupture of membranes: evaluating latency and neonatal morbidity for pregnancies with expectant management ≥34 weeks. J. Matern. Fetal Neonatal Med. 35, 2135–2148 (2022).
Gasparrini, A. J. et al. Antibiotic perturbation of the preterm infant gut microbiome and resistome. Gut Microbes 7, 443–449 (2016).
Sun, X. Z., Xiao, C., Yao, J. E. & Ling, C. Impact of postnatal exposure to antibiotics on intestinal microbiome in preterm infants. Chin. J. Perinat. Med. 12, 458–464 (2018).
Zou, Z. H. et al. Prenatal and postnatal antibiotic exposure influences the gut microbiota of preterm infants in neonatal intensive care units. Ann. Clin. Microbiol. Antimicrob. 17, 9 (2018).
Russell, J. T. et al. Antibiotics and the developing intestinal microbiome, metabolome and inflammatory environment in a randomized trial of preterm infants. Sci. Rep. 11, 1943 (2021).
Pammi, M. et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5, 31 (2017).
Strobel, K. M., Del Vecchio, G., Devaskar, S. U. & Calkins, K. L. Gut microbes and circulating cytokines in preterm infants with growth failure. J. Nutr. 153, 120–130 (2023).
Esmaeilizand, R. et al. Antibiotic exposure and development of necrotizing enterocolitis in very preterm neonates. Paediatr. Child Health 23, e56–e61 (2018).
Cotten, C. M. et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 123, 58–66 (2009).
Alexander, V. N., Northrup, V. & Bizzarro, M. J. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J. Pediatr. 159, 392–397 (2011).
Kuppala, V. S., Meinzen-Derr, J., Morrow, A. L. & Schibler, K. R. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J. Pediatr. 159, 720–725 (2011).
Abdel Ghany, E. A. & Ali, A. A. Empirical antibiotic treatment and the risk of necrotizing enterocolitis and death in very low birth weight neonates. Ann. Saudi Med. 32, 521–526 (2012).
Cantey, J. B., Pyle, A. K., Wozniak, P. S., Hynan, L. S. & Sanchez, P. J. Early antibiotic exposure and adverse outcomes in preterm, very low birth weight infants. J. Pediatr. 203, 62–67 (2018).
Reid, B. M., Eisenberg, R., Forman, K. & LaTuga, M. S. Relationship between early antibiotic exposure and short-term growth velocity in premature neonates. Am. J. Perinatol. 36, 1014–1022 (2019).
Krediet, T. G. et al. Microbiological factors associated with neonatal necrotizing enterocolitis: protective effect of early antibiotic treatment. Acta Paediatr. 92, 1180–1182 (2003).
Berkhout, D. J. C. et al. Risk factors for necrotizing enterocolitis: a prospective multicenter case-control study. Neonatology 114, 277–284 (2018).
Ting, J. Y. et al. Duration of initial empirical antibiotic therapy and outcomes in very low birth weight infants. Pediatrics 143, e20182286 (2019).
Reed, B. D., Schibler, K. R., Deshmukh, H., Ambalavanan, N. & Morrow, A. L. The impact of maternal antibiotics on neonatal disease. J. Pediatr. 197, 97–103.e103 (2018).
Juul, S. E. et al. A randomized trial of erythropoietin for neuroprotection in preterm infants. N. Engl. J. Med. 382, 233–243 (2020).
Bell, M. J. Neonatal necrotizing enterocolitis. N. Engl. J. Med. 298, 281–282 (1978).
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13, 59 (2013).
Goldberg, D. L. et al. Identifying malnutrition in preterm and neonatal populations: recommended indicators. J. Acad. Nutr. Diet. 118, 1571–1582 (2018).
Strobel, K. M. et al. Contemporary definitions of infant growth failure and neurodevelopmental and behavioral outcomes in extremely premature infants at two years of age. J. Perinatol. 44, 811–818 (2024).
Jensen, E. A. et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am. J. Respir. Crit. Care Med. 200, 751–759 (2019).
Zhang, Z. Survival analysis in the presence of competing risks. Ann. Transl. Med. 5, 47 (2017).
Puopolo, K. M. et al. Management of neonates born at ≤34 6/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics 142, e20182896 (2018).
Puopolo, K. M. et al. Identification of extremely premature infants at low risk for early-onset sepsis. Pediatrics 140, e20170925 (2017).
Hallaian, S. & Payton, K. Stratifying antibiotic use metrics by gestational age and first seven days optimizes antibiotic stewardship in neonatal intensive care units. J. Perinatol. 44, 116–118 (2024).
Jones, I. H. & Hall, N. J. Contemporary outcomes for infants with necrotizing enterocolitis – a systematic review. J. Pediatr. 220, 86–92.e83 (2020).
Bury, R. G. & Tudehope, D. Enteral antibiotics for preventing necrotizing enterocolitis in low birthweight or preterm infants. Cochrane Database Syst. Rev., CD000405 https://doi.org/10.1002/14651858.CD000405 (2001).
Masi, A. C. et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut 70, 2273–2282 (2021).
Sim, K. et al. Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clin. Infect. Dis. 60, 389–397 (2015).
Morrow, A. L. et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 1, 13 (2013).
Klopp, J. et al. Meconium microbiome of very preterm infants across Germany. mSphere 7, e0080821 (2022).
Morowitz, M. J. et al. The NICU Antibiotics and Outcomes (Nano) Trial: a randomized multicenter clinical trial assessing empiric antibiotics and clinical outcomes in newborn preterm infants. Trials 23, 428 (2022).
Wan, S. et al. Impact of exposure to antibiotics during pregnancy and infancy on childhood obesity: a systematic review and meta-analysis. Obesity 28, 793–802 (2020).
Solans, M., Barcelo, M. A., Morales-Suarez-Varela, M., Moya, A. & Saez, M. Prenatal exposure to antibiotics and risk of childhood overweight or obesity: a systematic review and meta-analysis. Obes. Rev. 23, e13382 (2022).
Zhou, P. et al. Perinatal antibiotic exposure affects the transmission between maternal and neonatal microbiota and is associated with early-onset sepsis. mSphere 5, e00984–19 (2020).
Muraoka, J., Kaneko, M., Doi, K., Kodama, Y. & Sameshima, H. Antepartum antibiotic therapy under 34 weeks of gestation and its impact on early-onset neonatal infection and maternal vaginal microbiota. Microbiol. Res. 13, 598–608 (2022).
Hermansson, H. et al. Breast milk microbiota is shaped by mode of delivery and intrapartum antibiotic exposure. Front. Nutr. 6, 4 (2019).
Coker, M. O. et al. Specific class of intrapartum antibiotics relates to maturation of the infant gut microbiota: a prospective cohort study. BJOG 127, 217–227 (2020).
Salas, A. A. et al. Risk assessment of cognitive impairment at 2 years of age in infants born extremely preterm using the intergrowth-21st growth standards. J. Pediatr. 275, 114239 (2024).
Lin, Y. C. et al. Association of neonatal antibiotic exposure with long-term growth trajectory faltering in preterm-birth children. Neonatology 121, 396–405 (2024).
Trasande, L. et al. Infant antibiotic exposures and early-life body mass. Int J. Obes. 37, 16–23 (2013).
Bailey, L. C. et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 168, 1063–1069 (2014).
Gerber, J. S. et al. Antibiotic exposure during the first 6 months of life and weight gain during childhood. JAMA 315, 1258–1265 (2016).
Younge, N. E. et al. Disrupted maturation of the microbiota and metabolome among extremely preterm infants with postnatal growth failure. Sci. Rep. 9, 8167 (2019).
Neves, L. L., Hair, A. B. & Preidis, G. A. A systematic review of associations between gut microbiota composition and growth failure in preterm neonates. Gut Microbes 15, 2190301 (2023).
Kumbhare, S. V. et al. Source of human milk (mother or donor) is more important than fortifier type (human or bovine) in shaping the preterm infant microbiome. Cell Rep. Med. 3, 100712 (2022).
Salas, A. A. et al. The gut microbiome of extremely preterm infants randomized to the early progression of enteral feeding. Pediatr. Res. 92, 799–804 (2022).
Nolan, L. S., Rimer, J. M. & Good, M. The role of human milk oligosaccharides and probiotics on the neonatal microbiome and risk of necrotizing enterocolitis: a narrative review. Nutrients 12, 3052 (2020).
Fenton, T. R. et al. “Extrauterine growth restriction” and “postnatal growth failure” are misnomers for preterm infants. J. Perinatol. 40, 704–714 (2020).
Salas, A. A., Bhatia, A. & Carlo, W. A. Postnatal growth of preterm infants 24 to 26 weeks of gestation and cognitive outcomes at 2 years of age. Pediatr. Res. 89, 1804–1809 (2021).
Rochow, N. et al. Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatr. Res. 79, 870–879 (2016).
Wood, A. J., Raynes-Greenow, C. H., Carberry, A. E. & Jeffery, H. E. Neonatal length inaccuracies in clinical practice and related percentile discrepancies detected by a simple length-board. J. Paediatr. Child Health 49, 199–203 (2013).
Acknowledgements
The PENUT Trial was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award numbers U01NS077953 and U01NS077955.
Author information
Authors and Affiliations
Contributions
K.M.S. conceptualized the study. K.M.S., T.R.W., S.E.J., G.C.V., K.M.P., and D.T.H. provided input in the study design. T.R.W. and K.M.S. performed the data analysis. K.M.S. wrote the original draft of the manuscript. T.R.W., G.C.V., K.M.P., O.B., D.T.H., D.E.M., M.P.D., P.J.H., and S.E.J. assisted with revisions to the manuscript. All authors approve the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The PENUT Trial was approved by the institutional review board at each participating site and was registered with the Food and Drug Administration. The study was performed in accordance with the Declaration of Helsinki.
Informed consent
Each participant participated with informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Strobel, K.M., Wood, T.R., Valentine, G.C. et al. Effect of early antibiotic exposure on necrotizing enterocolitis and growth in extremely preterm infants. Pediatr Res 98, 137–143 (2025). https://doi.org/10.1038/s41390-025-03928-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-025-03928-y
This article is cited by
-
Early antibiotic exposure and the risk of necrotizing enterocolitis in preterm neonates: insights from a large multicenter cohort in China
Pediatric Research (2025)
-
The double-edged sword of early antibiotic exposure in extremely preterm infants: implications for necrotizing enterocolitis and growth faltering
Pediatric Research (2025)