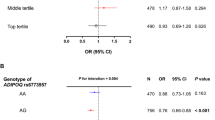
Abstract
Objectives
This meta-analysis aimed to assess circulating adipokine levels in girls with central precocious puberty (CPP) and compare them with those in healthy controls.
Methods
An exhaustive literature search was conducted, using the Embase, PubMed, Web of Science, Cochrane Library, and Scopus databases, from the inception of the study to October 31, 2023, to identify relevant studies. Studies comparing the serum levels of adiponectin, leptin, irisin, apelin, omentin,chemerin, resistin, vaspin, and visfatin in girls with CPP and healthy girls of the same age were included. The findings were summarized in Grading of Recommendations Assessment, Development, and Evaluation evidence profiles and synthesized qualitatively.
Results
Eleven studies that included 701 girls with CPP and 590 healthy girls were analyzed after the selection process. Leptin levels were significantly increased, whereas adiponectin levels were decreased in girls with CPP. Irisin levels did not change significantly. Subgroup and meta-regression analyses indicated that the heterogeneity in the association of leptin with CPP might be due to factors such as the number of cases, diagnostic criteria for CPP, and measurement methods.
Conclusions
Adipokines levels were altered in girls with CPP compared with those in healthy controls. Preventing obesity in children and adolescents with CPP is crucial.
PROSPERO registration number
CRD42022371490.
Impact
-
This meta-analysis is the first to explore the relationship between adipokines and central precocious puberty.
-
The results of this systematic review provide evidence that adipokines levels are altered in girls with central precocious puberty (CPP) compared with those in healthy controls. CPP in girls increases the risk of cardiovascular disease in adulthood. Preventing obesity in children and adolescents, especially in those with precocious puberty, is crucial.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets analyzed in the present study are available from the published papers that have been cited in this manuscript.
References
Latronico, A. C., Brito, V. N. & Carel, J. C. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol 4, 265–274 (2016).
Chen, M. & Eugster, E. A. Central precocious puberty: update on diagnosis and treatment. Paediatr Drugs 17, 273–281 (2015).
Brito, V. N. et al. Central precocious puberty: revisiting the diagnosis and therapeutic management. Arch Endocrinol Metab 60, 163–172 (2016).
Kim, Y. J. et al. Incidence and prevalence of central precocious puberty in Korea: an epidemiologic study based on a national database. J Pediatr 208, 221–228 (2019).
Bräuner, E. V. et al. Trends in the incidence of central precocious puberty and normal variant puberty among children in Denmark, 1998 to 2017. JAMA Netw Open 3, e2015665 (2020).
Eckert-Lind, C. et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: A systematic review and meta-analysis. JAMA Pediatr 174, e195881 (2020).
Li, H. et al. Changes in children’s healthcare visits during coronavirus disease-2019 pandemic in Hangzhou, China. J Pediatr 224, 146–149 (2020).
Stagi, S. et al. Increased incidence of precocious and accelerated puberty in females during and after the Italian lockdown for the coronavirus 2019 (COVID-19) pandemic. Ital J Pediatr 46, 165 (2020).
Mogensen, S. S. et al. Diagnostic work-up of 449 consecutive girls who were referred to be evaluated for precocious puberty. J Clin Endocrinol Metab 96, 1393–1401 (2011).
Parent, A. S. et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev 24, 668–693 (2003).
Massart, F. et al. High incidence of central precocious puberty in a bounded geographic area of northwest Tuscany: an estrogen disrupter epidemic? Gynecol Endocrinol 20, 92–98 (2005).
Luan, X. et al. Association study of the polymorphisms in the KISS1 gene with central precocious puberty in Chinese girls. Eur J Endocrinol 157, 113–118 (2007).
Aksglaede, L., Juul, A., Olsen, L. W. & Sørensen, T. I. Age at puberty and the emerging obesity epidemic. PLoS ONE 4, e8450 (2009).
De Leonibus, C. et al. Timing of puberty and physical growth in obese children: a longitudinal study in boys and girls. Pediatr Obes 9, 292–299 (2014).
Atay, Z. et al. The prevalence and risk factors of premature thelarche and pubarche in 4- to 8-year-old girls. Acta Paediatr 101, e71–e75 (2012).
Lakshman, R. et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab 94, 4953–4960 (2009).
Jacobsen, B. K., Oda, K., Knutsen, S. F. & Fraser, G. E. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the Adventist Health Study, 1976-88. Int J Epidemiol 38, 245–252 (2009).
Garn, S. M., LaVelle, M., Rosenberg, K. R. & Hawthorne, V. M. Maturational timing as a factor in female fatness and obesity. Am J Clin Nutr 43, 879–883 (1986).
Freedman, D. S. et al. The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa heart study. BMC Pediatr 3, 3 (2003).
van Lenthe, F. J., Kemper, C. G. & van Mechelen, W. Rapid maturation in adolescence results in greater obesity in adulthood: the Amsterdam Growth and Health Study. Am J Clin Nutr 64, 18–24 (1996).
Lakshman, R. et al. Association between age at menarche and risk of diabetes in adults: results from the EPIC-Norfolk cohort study. Diabetologia 51, 781–786 (2008).
Kivimäki, M. et al. Association of age at menarche with cardiovascular risk factors, vascular structure, and function in adulthood: the Cardiovascular Risk in Young Finns study. Am J Clin Nutr. 87, 1876–1882 (2008).
Frontini, M. G., Srinivasan, S. R. & Berenson, G. S. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord 27, 1398–1404 (2003).
Remsberg, K. E. et al. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the Fels Longitudinal Study. J Clin Endocrinol Metab 90, 2718–2724 (2005).
Steinberger, J. et al. Adiposity in childhood predicts obesity and insulin resistance in young adulthood. J Pediatr 138, 469–473 (2001).
Srinivasan, S. R., Myers, L. & Berenson, G. S. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes 51, 204–209 (2002).
Wahab, F., Shahab, M. & Behr, R. Hypothesis: Irisin is a metabolic trigger for the activation of the neurohormonal axis governing puberty onset. Med Hypotheses 95, 1–4 (2016).
Havel, P. J. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol 13, 51–59 (2002).
Hales, C. et al. Obesity, leptin and host defence of Streptococcus pneumoniae: the case for more human research. Eur Respir Rev 31, 220055 (2022).
Fang, H. & Judd, R. L. Adiponectin regulation and function. Compr Physiol 8, 1031–1063 (2018).
Park, K. H. et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab 98, 4899–4907 (2013).
Chen, T. et al. Serum CTRP3 levels in obese children: a potential protective adipokine of obesity, insulin sensitivity and pancreatic β cell function. Diabetes Metab Syndr Obes 12, 1923–1930 (2019).
Kutlu, E. et al. Serum irisin levels in central precocious puberty and its variants. J Clin Endocrinol Metab 106, e247–e254 (2021).
Kang, M. J. et al. The usefulness of circulating levels of leptin, kisspeptin, and neurokinin B in obese girls with precocious puberty. Gynecol Endocrinol 34, 627–630 (2018).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 (2009).
Chatenoud, L. & Vecchia, C. Comments: Meta-analysis of observational studies in epidemiology. Revue d Épidémiologie et de Santé Publique 48, 411–412 (2000).
Zhou, X. et al. Systematic review and meta-analysis on the effects of astaxanthin on human skin ageing. Nutrients 13, 2917 (2021).
Wang, R. et al. Stem cell therapy for Crohn’s disease: systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res Ther 12, 463 (2021).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605 (2010).
Hardy, R. J. & Thompson, S. G. Detecting and describing heterogeneity in meta‐analysis. Stat Med. 17, 841–856 (1998).
Huang, M. et al. The effect of gum consumption on anthropometric characteristics and cardiac disorders: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 54, 102578 (2020).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986).
Larmore, K. A. et al. Leptin and estradiol as related to change in pubertal status and body weight. Med Sci Mon 8, CR206–CR210 (2002).
Su, P. H. et al. A study of anthropomorphic and biochemical characteristics in girls with central precocious puberty and thelarche variant. J Pediatr Endocrinol Metabol 21, 213–220 (2008).
Pita, J. et al. Circulating kisspeptin levels exhibit sexual dimorphism in adults, are increased in obese prepubertal girls and do not suffer modifications in girls with idiopathic central precocious puberty. Peptides 32, 1781–1786 (2011).
Su, P. H. et al. Study of leptin levels and gene polymorphisms in patients with central precocious puberty. Pediatr Res. 71, 361–367 (2012).
Kang, M. J. et al. The influences of circulating leptin, kisspeptin, and neurokinin B levels to precocious puberty in obese girls. Hormone Res Paediatr 86, 419–419 (2016).
Sitticharoon, C. et al. Corrigendum to: Increased high molecular weight adiponectin, but decreased total adiponectin and kisspeptin, in central precocious puberty compared with aged-matched prepubertal girls. Reprod Fertil, Dev 29, 2506 (2017).
Zurita-Cruz, J. N. et al. Altered cardiometabolic profile in girls with central precocious puberty and adipokines: A propensity score matching analysis. Cytokine 148, 155660 (2021).
Chen, Y. et al. Serum irisin levels increase in girls with central precocious puberty not dependent on BMI: a pilot study. Endocrine Connect 11, e220028 (2022).
He, Z. & Yuan, B. Diagnostic value of combined detection of pelvic ultrasound and serum LH, FSH, and E2 Levels in children with idiopathic central precocious puberty. Evid Based Complement Alternat Med. 2022, 7928344 (2022).
Stöckl, D. et al. Age at menarche and its association with the metabolic syndrome and its components: results from the KORA F4 study. PLoS ONE 6, e26076 (2011).
Labayen, I. et al. The effect of early menarche on later body composition and fat distribution in female adolescents: role of birth weight. Ann Nutr Metab 54, 313–320 (2009).
Benedet, J. et al. Association of sexual maturation with excess body weight and height in children and adolescents. BMC Pediatr 14, 72 (2014).
Pinkney, J. et al. Adiposity, chronic inflammation, and the prepubertal decline of sex hormone binding globulin in children: evidence for associations with the timing of puberty (Earlybird 58). J Clin Endocrinol Metab 99, 3224–3232 (2014).
Lazar, L. et al. Treated and untreated women with idiopathic precocious puberty: BMI evolution, metabolic outcome, and general health between third and fifth decades. J Clin Endocrinol Metab 100, 1445–1451 (2015).
Woo, J. G. et al. Adolescent sex differences in adiponectin are conditional on pubertal development and adiposity. Obes Res. 13, 2095–2101 (2005).
Pilcová, R. et al. Leptin levels in obese children: effects of gender, weight reduction and androgens. Physiol Res. 52, 53–60 (2003).
Alikaşifoğlu, A. et al. The relationship between serum adiponectin, tumor necrosis factor-alpha, leptin levels and insulin sensitivity in childhood and adolescent obesity: adiponectin is a marker of metabolic syndrome. J Clin Res Pediatr Endocrinol 1, 233–239 (2009).
Vilariño-García, T. et al. Role of leptin in obesity, cardiovascular disease, and type 2 diabetes. Int J Mol Sci. 25, 2338 (2024).
Cunningham, M. J., Clifton, D. K. & Steiner, R. A. Leptin’s actions on the reproductive axis: perspectives and mechanisms. Biol Reprod 60, 216–222 (1999).
Dardeno, T. A. et al. Leptin in human physiology and therapeutics. Front Neuroendocrinol 31, 377–393 (2010).
Chou, S. H. & Mantzoros, C. 20 years of leptin: role of leptin in human reproductive disorders. J Endocrinol 223, T49–T62 (2014).
Palmert, M. R., Radovick, S. & Boepple, P. A. Leptin levels in children with central precocious puberty. J Clin Endocrinol Metab 83, 2260–2265 (1998).
Abacı, A. et al. Significance of serum neurokinin B and kisspeptin levels in the differential diagnosis of premature thelarche and idiopathic central precocious puberty. Peptides 64, 29–33 (2015).
Anubhuti & Arora, S. Leptin and its metabolic interactions: an update. Diabetes Obes Metab 10, 973–993 (2008).
Farooqi, I. S. O’Rahilly, S., 20 years of leptin: human disorders of leptin action. J Endocrinol 223, T63–T70 (2014).
Zurita-Cruz, J. N. et al. Relationship Between Serum Leptin Levels And Weight Gain In Girls With Central Precocious Puberty At 1-Year Follow-Up. Endocr Pract 23, 519–525 (2017).
Böttner, A. et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab 89, 4053–4061 (2004).
Aydin, S. et al. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides 61, 130–136 (2014).
Ruan, Q. et al. The effects of both age and sex on irisin levels in paired plasma and cerebrospinal fluid in healthy humans. Peptides 113, 41–51 (2019).
Elizondo-Montemayor, L. et al. Association of irisin plasma levels with anthropometric parameters in children with underweight, normal weight, overweight, and obesity. Biomed Res Int. 2017, 2628968 (2017).
Jang, H. B. et al. Association of circulating irisin levels with metabolic and metabolite profiles of Korean adolescents. Metabolism 73, 100–108 (2017).
Nigro, E. et al. Adiponectin profile and Irisin expression in Italian obese children: Association with insulin-resistance. Cytokine 94, 8–13 (2017).
Reinehr, T., Elfers, C., Lass, N. & Roth, C. L. Irisin and its relation to insulin resistance and puberty in obese children: a longitudinal analysis. J Clin Endocrinol Metab 100, 2123–2130 (2015).
Wahab, F. et al. Irisin in the primate hypothalamus and its effect on GnRH in vitro. J Endocrinol 241, 175–187 (2019).
Ulker, N. et al. Irisin may have a role in pubertal development and regulation of reproductive function in rats. Reproduction 160, 281–292 (2020).
Poretsky, L. et al. Reproductive effects of irisin: Initial in vitro studies. Reprod Biol 17, 285–288 (2017).
Alessandri, S. B. et al. Bone mineral density and body composition in girls with idiopathic central precocious puberty before and after treatment with a gonadotropin-releasing hormone agonist. Clinics 67, 591–596 (2012).
Chiocca, E. et al. Body mass index and body composition in adolescents treated with gonadotropin-releasing hormone analogue triptorelin depot for central precocious puberty: data at near final height. Neuroendocrinology 89, 441–447 (2009).
de Zegher, F., García Beltrán, C., López-Bermejo, A. & Ibáñez, L. Metformin for Rapidly Maturing Girls with Central Adiposity: Less Liver Fat and Slower Bone Maturation. Horm Res Paediatr 89, 136–140 (2018).
Bassols, J. et al. Effects of metformin administration on endocrine-metabolic parameters, visceral adiposity and cardiovascular risk factors in children with obesity and risk markers for metabolic syndrome: A pilot study. PLoS ONE 14, e0226303 (2019).
Mansfield, R. et al. Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril 79, 956–962 (2003).
Tosca, L., Chabrolle, C., Uzbekova, S. & Dupont, J. Effects of metformin on bovine granulosa cells steroidogenesis: possible involvement of adenosine 5’ monophosphate-activated protein kinase (AMPK). Biol Reprod 76, 368–378 (2007).
Velazquez, E. M., Mendoza, S. G., Wang, P. & Glueck, C. J. Metformin therapy is associated with a decrease in plasma plasminogen activator inhibitor-1, lipoprotein(a), and immunoreactive insulin levels in patients with the polycystic ovary syndrome. Metabolism 46, 454–457 (1997).
Glueck, C. J. et al. Hypofibrinolytic and atherogenic risk factors for stroke. J Lab Clin Med. 125, 319–325 (1995).
Acknowledgements
Not applicable.
Funding
Beijing University of Chinese Medicine vertical research and development fund (No. 2023-ZXFZJJ-058).
Author information
Authors and Affiliations
Contributions
MJ: scientific design, study selection, data extraction and manuscript writing. YG: literature search, study selection, data analysis and figure preparation. LH: study selection, data extraction and manuscript editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The research type of this article is systematic review and meta-analysis, thus no ethical approval is needed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, M., Gao, Y. & Huang, L. Circulating adipokines in girls with central precocious puberty: A systematic review and meta-analysis. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-03976-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-03976-4