Abstract
Background
To determine the association between exposure to Food and Drug Administration (FDA) warned anesthetics in premature infants and their full-scale intelligence quotient (FSIQ) score at 5 years of age.
Methods
Premature infants born <27 weeks gestational age (GA) between January 2006 and December 2012 with FDA anesthetic exposure status were included. Exposures included volatile anesthetics, propofol, benzodiazepines, ketamine, chloral hydrate, and barbiturates/phenobarbital. Exposure was treated as a binary variable with infants stratified into those who were or were not exposed to any FDA warned drug. Associations were explored using univariable and multivariable regressions.
Results
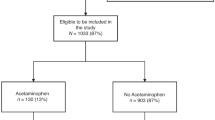
238 (61.5%) of 387 eligible infants had available FSIQ scores. Of these, 110 (46.2%) were exposed to warned anesthetics. Unadjusted and adjusted imputed case associations (95% CI) between FDA warned anesthetics and FSIQ were −5 (−10 to −2, p = 0.014) and −2 (−7 to 3, p = 0.528) points. An unobserved confounder(s) the strength of severe IVH [−9 points (−15 to −3)] would be required to overturn the directional association between FDA exposure and FSIQ in our complete case model.
Conclusion
Premature infants exposed to anesthetics flagged by the FDA showed no significant reduction in FSIQ at 5 years of age.
Impact
-
It is unclear whether early exposure to anesthetics in premature infants born <27 weeks gestation is associated with full-scale intelligence quotient (FSIQ) at 5 years of age.
-
Our retrospective cohort study included 387 premature infants born <27 weeks gestational age. FSIQ scores were available for 238/387 at 5 years of age of which 110 were exposed to Food and Drug Administration (FDA) warned anesthetic drugs.
-
After missing data imputation and adjustment for maternal and neonatal characteristics, no significant associations were found between FDA warned anesthetic exposure and FSIQ.
-
No adjusted volatile anesthetic or opioid dosage effect was associated with FSIQ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
In accordance with the Alberta Health Services Data Disclosure Agreement, we are not able to provide or make the data available for any purpose to a third party without the prior written consent of Alberta Health Services, Calgary, Alberta.
Change history
22 April 2025
The original online version of this article was revised: In the sentence beginning ‘We additionally included infant characteristics...’ in this article, the value ‘(BPD, NEC (≥stage 3)’ should have read ‘(BPD, NEC (≥stage 2)’.
23 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41390-025-04065-2
References
Lee, J. H., Zhang, J., Wei, L. & Yu, S. P. Neurodevelopmental implications of the general anesthesia in neonate and infants. Exp. Neurol. 272, 50–60 (2015).
Grover, L. A., Mitchell, R. B. & Szmuk, P. Anesthesia exposure and neurotoxicity in children - understanding the FDA warning and implications for the otolaryngologist. JAMA Otolaryngol. Neck Surg. 143, 1071 (2017).
Ho, A. M. H., Fleming, M. L. & Mizubuti, G. B. Anesthetic neurotoxicity and the developing brain. Can. Med. Assoc. J. 189, E1028–E1029 (2017).
Derderian, C. A., Szmuk, P. & Derderian, C. K. Behind the black box: the evidence for the U.S. Food and Drug Administration warning about the risk of general anesthesia in children younger than 3 years. Plast. Reconstr. Surg. 140, 787–792 (2017).
Jevtovic-Todorovic, V. et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 23, 876–882 (2003).
Ment, L. R., Hirtz, D. & Hüppi, P. S. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 8, 1042–1055 (2009).
Thompson, D. K. et al. Characterization of the corpus callosum in very preterm and full-term infants utilizing MRI. NeuroImage 55, 479–490 (2011).
Moser, J.J. et al. Association of sedation and anesthesia on cognitive outcomes in very premature infants: a retrospective observational study. Can. J. Anesth. 70, 56–68 (2023).
McCann, M. E. & Soriano, S. G. Does general anesthesia affect neurodevelopment in infants and children?. BMJ 367, l6459 (2019).
Ing, C. H. et al. Neurodevelopmental outcomes after initial childhood anesthetic exposure between ages 3 and 10 years. J. Neurosurg. Anesthesiol. 26, 377–386 (2014).
Graham, M. R. et al. Neurodevelopmental assessment in kindergarten in children exposed to general anesthesia before the age of 4 years. Anesthesiology 125, 667–677 (2016).
Vandenbroucke, J. P. et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 18, 805–835 (2007).
Wechsler, D. WPPSI-III. Wechsler Preschool and Primary Scale of Intelligence-third Edition (WPPSI-III) (The Psychological Corporation, 2002).
Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence-Fourth Edition (WPPSI-IV) (The Psychological Corporation, 2012).
Sansone, S. M. et al. Improving IQ measurement in intellectual disabilities using true deviation from population norms. J. Neurodev. Disord. 6, 16 (2014).
Palisano, R. et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med Child Neurol. 39, 214–223 (1997).
Shennan, A. T., Dunn, M. S., Ohlsson, A., Lennox, K. & Hoskins, E. M. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 82, 527–532 (1988).
International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch. Ophthalmol. 123, 991 (2005).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
Bell, M. J. et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
van Buuren, S., Boshuizen, H. C. & Knook, D. L. Multiple imputation of missing blood pressure covariates in survival analysis. Stat. Med 18, 681–694 (1999).
von Hippel, P. T. How many imputation do you need? A two-stage calculation using a quadratic rule. Socio. Methods Res 49, 699–718 (2020).
Stochero, E. M. L., Lúcio, A. D. & Jacobi, L. F. Data variability in the imputation quality of missing data. Acta Sci. Agron. 46, e66185 (2024).
Rix, A. & Du, J. miselect: variable selection for multiply imputed data. version 0.9.2. Available from: https://CRAN.Rproject.org/package=miselect (2024).
Kuhn, M. & Johnson, K. Applied Predictive Modeling (Springer, 2013).
Gelman, A. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 27, 2865–2873 (2008).
Cinelli, C., Ferwerda, J. & Hazlett C. sensemakr: sensitivity analysis tools for regression models. p. 0.1.4. Available from: https://CRAN.R-project.org/package=sensemakr (2019).
McCann, M. E. et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet 393, 664–677 (2019).
Crilly, C. J., Haneuse, S. & Litt, J. S. Predicting the outcomes of preterm neonates beyond the neonatal intensive care unit: what are we missing?. Pediatr. Res. 89, 426–445 (2021).
Ing, C. et al. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics 130, e476–e485 (2012).
Gano, D. et al. Impaired cognitive performance in premature newborns with two or more surgeries prior to term-equivalent age. Pediatr. Res. 78, 323–329 (2015).
Reighard, C. et al. Anesthetic exposure during childhood and neurodevelopmental outcomes: a systematic review and meta-analysis. JAMA Netw. Open 5, e2217427 (2022).
Sun, L. S. et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA 315, 2312–2320 (2016).
Walkden, G. J. et al. Early childhood general anesthesia and neurodevelopmental outcomes in the Avon longitudinal study of parents and children birth cohort. Anesthesiology 133, 1007–1020 (2020).
Walkden, G. J., Pickering, A. E. & Gill, H. Assessing long-term neurodevelopmental outcome following general anesthesia in early childhood: challenges and opportunities. Anesth. Analg. 128, 681–694 (2019).
Rozé, J. C. et al. Prolonged sedation and/or analgesia and 5-year neurodevelopment outcome in very preterm infants: results from the EPIPAGE cohort. Arch. Pediatr. Adolesc. Med. 162, 728 (2008).
O’Leary, J. D. et al. A population-based study evaluating the association between surgery in early life and child development at primary school entry. Anesthesiology 125, 272–279 (2016).
Glatz, P. et al. Association of anesthesia and surgery during childhood with long-term academic performance. JAMA Pediatr. 171, e163470 (2017).
Puia-Dumitrescu, M. et al. Assessment of 2-year neurodevelopmental outcomes in extremely preterm infants receiving opioids and benzodiazepines. JAMA Netw. Open. 4, e2115998 (2021).
Acknowledgements
This work was supported by the Canadian Anesthesia Research Foundation as part of a Canadian Anesthesiologists’ Society Resident Research Grant awarded to Dr. Joanna Moser.
Author information
Authors and Affiliations
Contributions
J.J. Fifen drafted the initial version of the manuscript. M. Siddique and A. Lodha were involved in critical review and revision of the manuscript. J.J. Fifen, A. Lodha, D. McAllister, and A. Benlamri conceptualized and designed the study, were involved in the acquisition and interpretation of the data, and critical review and revision of the manuscript. A. Walker was involved in data acquisition, statistical analysis and interpretation of the data and critical review and revision of the manuscript. S. Tang was involved in the acquisition and interpretation of the data and critical review and revision of the manuscript. S. Makarchuk completes neurodevelopment assessments of the premature infants seen in the neonatal follow-up clinic and was involved in critical review and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
The Conjoint Health Research Ethics Board (CHREB ID: REB16-0632, University of Calgary, Calgary, Alberta, Canada) granted the researchers a waiver of consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the sentence beginning ‘We additionally included infant characteristics...’ in this article, the value ‘(BPD, NEC (≥stage 3)’ should have read ‘(BPD, NEC (≥stage 2)’.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fifen, J.J., Siddique, M., Lodha, A. et al. Anesthesia, extremely premature infants and full-scale intelligence quotient at 5 years of age. Pediatr Res 98, 1904–1911 (2025). https://doi.org/10.1038/s41390-025-04023-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-025-04023-y