Abstract
Background
Necrotizing enterocolitis (NEC) is mediated by toll-like receptor 4 (TLR4)-induced inflammation and is preceded by reduced intestinal motility. Human milk oligosaccharides (HMOs) are non-digestible components of breast milk that prevent NEC in preclinical models. We now hypothesize that HMOs can reduce the risk of NEC through restoration of intestinal motility and reduced TLR4-mediated inflammation.
Methods
NEC was induced in C57-BL/6 mice through the combination of formula gavage, hypoxia, and oral administration of NEC stool. Mice were administered either 2’-FL (5 g/L), 6’-SL (5 g/L), or a blend of 5 specific HMOs (5 g/L) containing 2’-FL (2.606 g/L), 3’-FL (0.652 g/L), LNT (1.304 g/L), 3’-SL (0.174 g/L), and 6’-SL (0.260 g/L). Gastrointestinal motility was assessed by 70 Kd FITC-dextran transit time. Enteric glia were quantified by immunohistochemistry and qRT-PCR expression.
Results
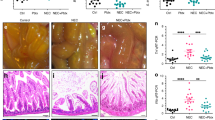
Administration of either 2’-FL, 6’-SL, or HMO blend significantly attenuated NEC severity and reversed intestinal hypomotility. HMOs prevented enteric glia loss and regulated key genes critical for enteric glia maintenance, attenuated pro-apoptotic genes, and increased anti-apoptotic genes in vitro, resulting in a reduction in apoptosis. Strikingly, HMOs reduced LPS-TLR4-induced NFκB signaling and ROS generation in enteric glia.
Conclusions
HMOs protect against NEC at least in part through protective effects on inflammation and the enteric nervous system.
Impact
-
This study sheds light on the role of certain human milk oligosaccharides in a clinically relevant mouse model of NEC and adds additional insights into their underlying mechanism of action by revealing a protective effect on the enteric nervous system.
-
These results reveal that HMOs prevent the loss of enteric glia in NEC and influence the expression of genes that regulate enteric glia maintenance.
-
HMOs also limit TLR4-NFkB signaling, providing an additional mechanism of enteric glia maintenance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All materials described in the manuscript, including all relevant raw data, are freely available to any researcher wishing to use them for non-commercial purposes after appropriate material transfer agreements.
References
Duess, J. W. et al. Necrotizing enterocolitis, gut microbes, and sepsis. Gut Microbes 15, 2221470 (2023).
Hackam, D. J. & Sodhi, C. P. Toll-like receptor-mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell Mol. Gastroenterol. Hepatol. 6, 229–238 e221 (2018).
Hackam, D. J. & Sodhi, C. P. Bench to bedside - new insights into the pathogenesis of necrotizing enterocolitis. Nat. Rev. Gastroenterol. Hepatol. 19, 468–479 (2022).
Scheese, D. J., Sodhi, C. P. & Hackam, D. J. New insights into the pathogenesis of necrotizing enterocolitis and the dawn of potential therapeutics. Semin. Pediatr. Surg. 32, 151309 (2023).
Holman, R. C. et al. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr. Perinat. Epidemiol. 20, 498–506 (2006).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Samuels, N., van de Graaf, R. A., de Jonge, R. C. J., Reiss, I. K. M. & Vermeulen, M. J. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr. 17, 105 (2017).
Cai, X., Liebe, H. L., Golubkova, A., Leiva, T. & Hunter, C. J. A review of the diagnosis and treatment of necrotizing enterocolitis. Curr. Pediatr. Rev. 19, 285–295 (2023).
Hackam, D. J., Sodhi, C. P. & Good, M. New insights into necrotizing enterocolitis: from laboratory observation to personalized prevention and treatment. J. Pediatr. Surg. 54, 398–404 (2019).
Roberts, A. G., Younge, N. & Greenberg, R. G. Neonatal necrotizing enterocolitis: an update on pathophysiology, treatment, and prevention. Paediatr. Drugs 26, 259–275 (2024).
Thurl, S., Munzert, M., Boehm, G., Matthews, C. & Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 75, 920–933 (2017).
Autran, C. A. et al. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 67, 1064–1070 (2018).
Masi, A. C. et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut 70, 2273–2282 (2021).
Sodhi, C. P. et al. The human milk oligosaccharides 2’-fucosyllactose and 6’-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr. Res. 89, 91–101 (2021).
Hackam, D. J., Afrazi, A., Good, M. & Sodhi, C. P. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clin. Dev. Immunol. 2013, 475415 (2013).
Sampath, V. et al. Sigirr genetic variants in premature infants with necrotizing enterocolitis. Pediatrics 135, e1530–e1534 (2015).
Neal, M. D. et al. Discovery and validation of a new class of small molecule toll-like receptor 4 (TLR4) inhibitors. PLoS ONE 8, e65779 (2013).
Kovler, M. L. et al. Toll-like receptor 4-mediated enteric glia loss is critical for the development of necrotizing enterocolitis. Sci. Transl. Med. 13, eabg3459 (2021).
Hill, D. R., Chow, J. M. & Buck, R. H. Multifunctional benefits of prevalent hmos: implications for infant health. Nutrients 13, 3364 (2021).
Good, M. et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 8, 1166–1179 (2015).
Good, M., Sodhi, C. P. & Hackam, D. J. Evidence-based feeding strategies before and after the development of necrotizing enterocolitis. Expert Rev. Clin. Immunol. 10, 875–884 (2014).
Sodhi, C. P. et al. Intestinal epithelial Tlr-4 activation is required for the development of acute lung injury after trauma/hemorrhagic shock via the release of Hmgb1 from the gut. J. Immunol. 194, 4931–4939 (2015).
Hoshiba, J. Method for hand-feeding mouse pups with nursing bottles. Contemp. Top. Lab. Anim. Sci. 43, 50–53 (2004).
Afrazi, A. et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J. Biol. Chem. 289, 9584–9599 (2014).
Good, M. et al. The human milk oligosaccharide 2’-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br. J. Nutr. 116, 1175–1187 (2016).
Halpern, M. D. et al. Apical sodium-dependent bile acid transporter upregulation is associated with necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G623–G631 (2010).
Sodhi, C. P. et al. Fat composition in infant formula contributes to the severity of necrotising enterocolitis. Br. J. Nutr. 120, 665–680 (2018).
Yazji, I. et al. Endothelial Tlr4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via enos-no-nitrite signaling. Proc. Natl. Acad. Sci. USA 110, 9451–9456 (2013).
Li, Z. et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 31, 8998–9009 (2011).
Miller, M. S., Galligan, J. J. & Burks, T. F. Accurate measurement of intestinal transit in the rat. J. Pharm. Methods 6, 211–217 (1981).
Sodhi, C. P. et al. Fat composition in infant formula contributes to the severity of necrotising enterocolitis. Br J. Nutr. 120, 665–680 (2018).
Hansebout, C. R. et al. Enteric Glia mediate neuronal outgrowth through release of neurotrophic factors. Neural Regen. Res. 7, 2165–2175 (2012).
Hoehner, J. C., Wester, T., Pahlman, S. & Olsen, L. Localization of neurotrophins and their high-affinity receptors during human enteric nervous system development. Gastroenterology 110, 756–767 (1996).
Zhou, Y. et al. Enteric nervous system abnormalities are present in human necrotizing enterocolitis: potential neurotransplantation therapy. Stem Cell Res. Ther. 4, 157 (2013).
Klein, S. et al. Interstitial cells of cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat. Commun. 4, 1630 (2013).
Chow, A. K. & Gulbransen, B. D. Potential roles of enteric glia in bridging neuroimmune communication in the Gut. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G145–G152 (2017).
Bassotti, G. et al. Enteric neuroglial apoptosis in inflammatory bowel diseases. J. Crohns Colitis 3, 264–270 (2009).
Villanacci, V. et al. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol. Motil. 20, 1009–1016 (2008).
Ghoshal, U. C. Marshall and Warren lecture 2019: a paradigm shift in pathophysiological basis of irritable bowel syndrome and its implication on treatment. J. Gastroenterol. Hepatol. 35, 712–721 (2020).
Sperber, A. D. et al. Greater overlap of ROME IV disorders of gut-brain interactions leads to increased disease severity and poorer quality of life. Clin. Gastroenterol. Hepatol. 20, e945–e956 (2022).
Neumann, C. J. et al. Clinical nec prevention practices drive different microbiome profiles and functional responses in the preterm intestine. Nat. Commun. 14, 1349 (2023).
Wejryd, E. et al. Low diversity of human milk oligosaccharides is associated with necrotising enterocolitis in extremely low birth weight infants. Nutrients 10, 1556 (2018).
Bering, S. B. Human milk oligosaccharides to prevent gut dysfunction and necrotizing enterocolitis in preterm neonates. Nutrients 10, 1461 (2018).
Nolan, L. S., Rimer, J. M. & Good, M. The role of human milk oligosaccharides and probiotics on the neonatal microbiome and risk of necrotizing enterocolitis: a narrative review. Nutrients 12, 3052 (2020).
Wang, C. et al. Human milk oligosaccharides protect against necrotizing enterocolitis by inhibiting intestinal damage via increasing the proliferation of crypt cells. Mol. Nutr. Food Res. 63, e1900262 (2019).
Moukarzel, S. & Bode, L. Human milk oligosaccharides and the preterm infant: a journey in sickness and in health. Clin. Perinatol. 44, 193–207 (2017).
Vandenplas, Y. et al. Human milk oligosaccharides: 2’-fucosyllactose (2’-Fl) and lacto-N-neotetraose (Lnnt) in infant formula. Nutrients 10, 1161 (2018).
Sodhi, C. P. et al. Human milk oligosaccharides reduce necrotizing enterocolitis-induced neuroinflammation and cognitive impairment in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 325, G23–G41 (2023).
Abbas, S. et al. Tailoring human milk oligosaccharides to prevent necrotising enterocolitis among preterm infants. Front. Nutr. 8, 702888 (2021).
Neal, M. D. et al. Enterocyte Tlr4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 176, 3070–3079 (2006).
Jilling, T. et al. The roles of bacteria and Tlr4 in rat and murine models of necrotizing enterocolitis. J. Immunol. 177, 3273–3282 (2006).
Lopez, C. M. et al. Models of necrotizing enterocolitis. Semin. Perinatol. 47, 151695 (2023).
Sodhi, C., Richardson, W., Gribar, S. & Hackam, D. J. The development of animal models for the study of necrotizing enterocolitis. Dis. Model. Mech. 1, 94–98 (2008).
Sodhi, C. P. et al. Intestinal epithelial toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143, 708–718.e705 (2012).
Lu, P. et al. Animal models of gastrointestinal and liver diseases. animal models of necrotizing enterocolitis: pathophysiology, translational relevance, and challenges. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G917–G928 (2014).
Sangild, P. T. et al. Preterm birth affects the intestinal response to parenteral and enteral nutrition in newborn pigs. J. Nutr. 132, 3786–3794 (2002).
Sangild, P. T. et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130, 1776–1792 (2006).
Cannavo, L. et al. Oxidative stress and respiratory diseases in preterm newborns. Int. J. Mol. Sci. 22, 12504 (2021).
Funding
This study was supported in part by a sponsored research grant from Abbott Nutrition. D.J.H. is supported by R35 GM141956, and C.T., and D.S. are supported by T32DK007713.
Author information
Authors and Affiliations
Contributions
D.J.H., C.P.S., D.J.S., C.T., W.B.F., J.W.D., K.T., M.E.S., R.H.B., D.R.H., A.S., T.P., S.W., and M.W. made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. D.J.H., C.P.S., R.H.B., D.R.H., and A.S. drafted the article or revised it critically for important intellectual content, and all authors gave their final approval of the version to be published.
Corresponding authors
Ethics declarations
Competing interests
R.H.B., D.R.H., and A.S.-D. are employees of Abbott, Nutrition Division, which supplied the products used in the current study and which provided funding for the current study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sodhi, C.P., Scheese, D.J., Tragesser, C. et al. Necrotizing enterocolitis: specific human milk oligosaccharides prevent enteric glia loss and hypomotility. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04077-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-04077-y