Abstract
This review explores the association between the NOD-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasomes and necrotizing enterocolitis (NEC) in neonates. It underscores the role of NLRP3 inflammasome overactivation in NEC pathogenesis and proposes that inhibiting NLRP3 inflammasome activation may improve therapeutic benefits. This review examines various strategies to inhibit NLRP3 inflammasome activation, detailing their pharmacological mechanisms.
Impact
-
The aim of this review is to discuss the role of NLRP3 inflammasome in the progression of NEC.
-
Abnormal NLRP3 inflammasome activation in patients with NEC and experimental models.
-
Molecules and proteins have been identified as regulators of NLRP3 inflammasome activity, offering avenues for NEC treatment.
-
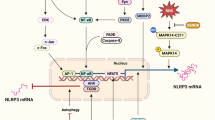
The NLRP3 inflammasome is a key contributor to NEC.
-
Novel NLRP3 inflammasome inhibitors are the focus of the development of NEC therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All the data analyzed in this review are included in this article and/or its figures. Further inquiries can be directed to the corresponding author.
References
Yee, W. H. et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 129, e298–e304 (2012).
Sarafidis, K., Agakidou, E., Kontou, A., Agakidis, C. & Neu, J. Struggling to understand the NEC spectrum—could the integration of metabolomics, clinical-laboratory data, and other emerging technologies help diagnosis? Metabolites 14, 521 (2024).
Patole, S. Microbiota and Necrotizing Enterocolitis. Nestle Nutr. Inst. Workshop Ser. 88, 81–94 (2017).
Sodhi, C. P. et al. The human milk oligosaccharides 2’-fucosyllactose and 6’-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr. Res. 89, 91–101 (2021).
Liu, T. et al. Toll-like receptor 4-mediated necroptosis in the development of necrotizing enterocolitis. Pediatr. Res. 91, 73–82 (2022).
Olaloye, O. O. et al. CD16+CD163+ monocytes traffic to sites of inflammation during necrotizing enterocolitis in premature infants. J. Exp. Med. 218, e20200344 (2021).
Hackam, D. J. & Sodhi, C. P. Toll-Like receptor-mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell Mol. Gastroenterol. Hepatol. 6, 229–238.e1 (2018).
Gomart, A., Vallée, A. & Lecarpentier, Y. Necrotizing enterocolitis: LPS/TLR4-induced crosstalk between canonical TGF-β/Wnt/β-catenin pathways and PPARγ. Front. Pediatr. 9, 713344 (2021).
Pan, H.-X. et al. Mucin 1 and interleukin-11 protein expression and inflammatory reactions in the intestinal mucosa of necrotizing enterocolitis children after surgery. World J. Clin. Cases 9, 7372–7380 (2021).
Huo, R., Liu, H., Chen, J., Sheng, H. & Miao, L. Serum HMGB1 level is correlated with serum I-FABP level in neonatal patients with necrotizing enterocolitis. BMC Pediatr. 21, 355 (2021).
Meyer, C. M., Khan, A. M. & Alcorn, J. L. Impact of surfactant protein-A on immunomodulatory properties of murine and human breast milk. J. Pediatr. Gastroenterol. Nutr. 75, 97–103 (2022).
Church, L. D., Cook, G. P. & McDermott, M. F. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat. Clin. Pr. Rheumatol. 4, 34–42 (2008).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Nanthakumar, N. et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One 6, e17776 (2011).
Guo, S. et al. Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88. J. Immunol. 195, 4999–5010 (2015).
Niño, D. F., Sodhi, C. P. & Hackam, D. J. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 13, 590–600 (2016).
Jarczak, D. & Nierhaus, A. Cytokine Storm-Definition, Causes, and Implications. Int J. Mol. Sci. 23, 11740 (2022).
Kuypers, F. A. Hyperinflammation, apoptosis, and organ damage. Exp. Biol. Med. (Maywood) 247, 1112–1123 (2022).
Coll, R. C. & Schroder, K. Inflammasome components as new therapeutic targets in inflammatory disease. Nat. Rev. Immunol. 25, 22–41 (2025).
Seplovich, G. et al. Inflammasome links traumatic brain injury, chronic traumatic encephalopathy, and Alzheimer’s disease. Neural Regen. Res. 20, 1644–1664 (2025).
Ball, D. P. et al. Caspase-1 interdomain linker cleavage is required for pyroptosis. Life Sci. Alliance 3, e202000664 (2020).
Ma, X. et al. LPS mediates bovine endometrial epithelial cell pyroptosis directly through both NLRP3 classical and non-classical inflammasome pathways. Front Immunol. 12, 676088 (2021).
Ji, Y., Hua, H., Jia, Z., Zhang, A. & Ding, G. Therapy Targeted to the NLRP3 Inflammasome in Chronic Kidney Disease. Kidney Dis. (Basel) 10, 369–383 (2024).
Vande Walle, L. & Lamkanfi, M. Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets. Nat. Rev. Drug Discov. 23, 43–66 (2024).
Voet, S., Srinivasan, S., Lamkanfi, M. & van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 11, e10248 (2019).
Hsu, C.-C. et al. Hematopoietic NLRP3 and AIM2 inflammasomes promote diabetes-accelerated atherosclerosis, but increased necrosis is independent of pyroptosis. Diabetes 72, 999–1011 (2023).
Bauernfeind, F. G. et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Xiao, L., Magupalli, V. G. & Wu, H. Cryo-EM structures of the active NLRP3 inflammasome disc. Nature 613, 595–600 (2023).
Fu, J. & Wu, H. Structural Mechanisms of NLRP3 inflammasome assembly and activation. Annu. Rev. Immunol. 41, 301–316 (2023).
Forlani, G., Shallak, M., Gatta, A., Shaik, A. K. B. & Accolla, R. S. The NLR member CIITA: master controller of adaptive and intrinsic immunity and unexpected tool in cancer immunotherapy. Biomed. J. 46, 100631 (2023).
He, Y., Hara, H. & Núñez, G. Mechanism and regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 41, 1012–1021 (2016).
Cheng, Z. et al. HECTD3 inhibits NLRP3 inflammasome assembly and activation by blocking NLRP3-NEK7 interaction. Cell Death Dis. 15, 86 (2024).
Zheng, S. et al. ZDHHC5-mediated NLRP3 palmitoylation promotes NLRP3-NEK7 interaction and inflammasome activation. Mol. Cell 83, 4570–4585.e7 (2023).
Mu, X. et al. Pyroptosis and inflammasomes in diabetic wound healing. Front Endocrinol. (Lausanne) 13, 950798 (2022).
Yang, X. et al. Piceatannol protects against age-related hearing loss by inhibiting cellular pyroptosis and inflammation through regulated Caspase11-GSDMD pathway. Biomed. Pharmacother. 163, 114704 (2023).
Xiaodong, L. & Xuejun, X. GSDMD-mediated pyroptosis in retinal vascular inflammatory diseases: a review. Int Ophthalmol. 43, 1405–1411 (2023).
Akbal, A. et al. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell Mol. Immunol. 19, 1201–1214 (2022).
Chen, J. & Chen, Z. J. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature 564, 71–76 (2018).
Daussy, C. F. et al. The inflammasome components NLRP3 and ASC act in concert with IRGM to rearrange the golgi apparatus during Hepatitis C virus infection. J. Virol. 95, e00826-20 (2021).
Zhang, Z. et al. Distinct changes in endosomal composition promote NLRP3 inflammasome activation. Nat. Immunol. 24, 30–41 (2023).
Tang, J. et al. Sequential ubiquitination of NLRP3 by RNF125 and Cbl-b limits inflammasome activation and endotoxemia. J. Exp. Med. 217, e20182091 (2020).
Xu, T. et al. Ubiquitination of NLRP3 by gp78/Insig-1 restrains NLRP3 inflammasome activation. Cell Death Differ. 29, 1582–1595 (2022).
Nie, L. et al. Consecutive palmitoylation and phosphorylation orchestrates NLRP3 membrane trafficking and inflammasome activation. Mol. Cell 84, 3336–3353.e7 (2024).
Niu, T. et al. NLRP3 phosphorylation in its LRR domain critically regulates inflammasome assembly. Nat. Commun. 12, 5862 (2021).
Qin, Y. et al. TRIM28 SUMOylates and stabilizes NLRP3 to facilitate inflammasome activation. Nat. Commun. 12, 4794 (2021).
Yoon, S.-J. et al. Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochem Biophys. Res Commun. 463, 1184–1189 (2015).
Li, Z. et al. S-glutathionylation in hepatocytes is involved in arsenic-induced liver fibrosis through activation of the NLRP3 inflammasome, an effect alleviated by NAC. Sci. Total Environ. 947, 174534 (2024).
Zhang, Y. et al. Acetylation is required for full activation of the NLRP3 inflammasome. Nat. Commun. 14, 8396 (2023).
Wei, W. et al. Sodium Tanshinone IIA Sulfonate alleviates vascular senescence in diabetic mice by modulating the A20-NFκB-NLRP3 inflammasome-catalase pathway. Sci. Rep. 14, 17665 (2024).
Chen, L. et al. Ursolic acid alleviates lupus nephritis by suppressing SUMO1-mediated stabilization of NLRP3. Phytomedicine 130, 155556 (2024).
Shao, L. et al. SUMO1 SUMOylates and SENP3 deSUMOylates NLRP3 to orchestrate the inflammasome activation. FASEB J. 34, 1497–1515 (2020).
Yang, M. et al. HDAC10 switches NLRP3 modification from acetylation to ubiquitination and attenuates acute inflammatory diseases. Cell Commun. Signal 22, 615 (2024).
Higashikuni, Y. et al. NLRP3 inflammasome activation through heart-brain interaction initiates cardiac inflammation and hypertrophy during pressure Overload. Circulation 147, 338–355 (2023).
Suetomi, T. et al. Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca 2+ /calmodulin-dependent protein kinase ii δ signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation 138, 2530–2544 (2018).
Fidler, T. P. et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 592, 296–301 (2021).
Terzioglu, G. & Young-Pearse, T. L. Microglial function, INPP5D/SHIP1 signaling, and NLRP3 inflammasome activation: implications for Alzheimer’s disease. Mol. Neurodegener. 18, 89 (2023).
Heneka, M. T. et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013).
Panicker, N. et al. Neuronal NLRP3 is a parkin substrate that drives neurodegeneration in Parkinson’s disease. Neuron 110, 2422–2437.e9 (2022).
Govindarajan, V., de Rivero Vaccari, J. P. & Keane, R. W. Role of inflammasomes in multiple sclerosis and their potential as therapeutic targets. J. Neuroinflamm. 17, 260 (2020).
Malhotra, S. et al. Increased NLRP3 INflammasome Activation and Pyroptosis in Patients with Multiple Sclerosis with Fingolimod Treatment Failure. Neurol. Neuroimmunol. Neuroinflamm.10, e200100 (2023).
Peelen, E. et al. Increased inflammasome related gene expression profile in PBMC may facilitate T helper 17 cell induction in multiple sclerosis. Mol. Immunol. 63, 521–529 (2015).
He, M. et al. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metab. 31, 580–591.e5 (2020).
Kim, S. R. et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 11, 2127 (2020).
Zhang, J. et al. ChemR23 signaling ameliorates cognitive impairments in diabetic mice via dampening oxidative stress and NLRP3 inflammasome activation. Redox Biol. 58, 102554 (2022).
Shi, B. et al. NLRP3 activation in macrophages promotes acute intestinal injury in neonatal necrotizing enterocolitis. World J. Pediatr. 20, 153–164 (2024).
Zhu, F. et al. Blockage of NLRP3 inflammasome activation ameliorates acute inflammatory injury and long-term cognitive impairment induced by necrotizing enterocolitis in mice. J. Neuroinflamm.18, 66 (2021).
Zhang, W., He-Yang, J., Tu, W. & Zhou, X. Sialylated human milk oligosaccharides prevent intestinal inflammation by inhibiting toll like receptor 4/NLRP3 inflammasome pathway in necrotizing enterocolitis rats. Nutr. Metab. (Lond.) 18, 5 (2021).
Hu, D. & Liu, H. Pyroptosis is involved in the pathogenesis of necrotizing enterocolitis]. Xi Bao Yu Fen. Zi Mian Yi Xue Za Zhi 34, 1070–1074 (2018).
Chen, Z. et al. Cronobacter sakazakii induces necrotizing enterocolitis by regulating NLRP3 inflammasome expression via TLR4. J. Med Microbiol 69, 748–758 (2020).
Leaphart, C. L. et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J. Immunol. 179, 4808–4820 (2007).
Shen, C. et al. Microbe-derived antioxidants protect ipec-1 cells from H2O2-induced oxidative stress, inflammation and tight junction protein disruption via activating the Nrf2 pathway to inhibit the ROS/NLRP3/IL-1β signaling pathway. Antioxidants 13, 533 (2024).
Chung, H. et al. NLRP3 regulates a non-canonical platform for caspase-8 activation during epithelial cell apoptosis. Cell Death Differ. 23, 1331–1346 (2016).
Yang, J. & Shi, Y. Paneth cell development in the neonatal gut: pathway regulation, development, and relevance to necrotizing enterocolitis. Front. Cell Dev. Biol. 11, 1184159 (2023).
Chen, L. et al. Microbiota metabolite butyrate differentially regulates Th1 and Th17 cells’ differentiation and function in induction of colitis. Inflamm. Bowel Dis. 25, 1450–1461 (2019).
Maheshwari, A. et al. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 140, 242–253 (2011).
Kovler, M. L. et al. Toll-like receptor 4-mediated enteric glia loss is critical for the development of necrotizing enterocolitis. Sci. Transl. Med. 13, eabg3459 (2021).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
Coll, R. C. et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 15, 556–559 (2019).
Tapia-Abellán, A. et al. MCC950 closes the active conformation of NLRP3 to an inactive state. Nat. Chem. Biol. 15, 560–564 (2019).
Feng, H. et al. Design, synthesis and biological evaluation of sulfonylurea derivatives as NLRP3 inflammasome inhibitors. Bioorg. Med. Chem. Lett. 114, 129987 (2024).
Zheng, Y. et al. MCC950 as a promising candidate for blocking NLRP3 inflammasome activation: A review of preclinical research and future directions. Arch. Pharm. (Weinh. 357, e2400459 (2024).
Chen, R., Kang, R. & Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 54, 91–102 (2022).
Yang, H., Wang, H. & Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 11, 484 (2020).
Tan, S.-W. et al. HMGB1 mediates cognitive impairment caused by the NLRP3 inflammasome in the late stage of traumatic brain injury. J. Neuroinflamm. 18, 241 (2021).
Yu, R. et al. Inhibition of HMGB1 improves necrotizing enterocolitis by inhibiting NLRP3 via TLR4 and NF-κB signaling pathways. J. Cell Physiol. 234, 13431–13438 (2019).
Zhang, W. et al. Bacteroides fragilis strain ZY-312 facilitates colonic mucosa regeneration in colitis via motivating STAT3 signaling pathway induced by IL-22 from ILC3 secretion. Front Immunol. 14, 1156762 (2023).
Zhou, Q. et al. Bacteroides fragilis strain ZY-312 promotes intestinal barrier integrity via upregulating the STAT3 pathway in a radiation-induced intestinal injury mouse model. Front Nutr. 9, 1063699 (2022).
Fan, H. et al. Bacteroides fragilis Strain ZY-312 Defense against Cronobacter sakazakii-Induced Necrotizing Enterocolitis In Vitro and in a Neonatal Rat Model. mSystems 4, e00305-19 (2019).
Ma, F. et al. Melatonin ameliorates necrotizing enterocolitis by preventing Th17/Treg imbalance through activation of the AMPK/SIRT1 pathway. Theranostics 10, 7730–7746 (2020).
Gao, X. et al. Melatonin-induced lncRNA LINC01512 prevents Treg/Th17 imbalance by promoting SIRT1 expression in necrotizing enterocolitis. Int Immunopharmacol. 96, 107787 (2021).
Lai, J. et al. Melatonin alleviates necrotizing enterocolitis by reducing bile acid levels through the SIRT1/FXR signalling axis. Int Immunopharmacol. 128, 111360 (2024).
Marseglia, L. et al. Oxidative stress-mediated damage in newborns with necrotizing enterocolitis: a possible role of melatonin. Am. J. Perinatol. 32, 905–909 (2015).
Xiong, X., Bao, Z., Mi, Y., Wang, X. & Zhu, J. melatonin alleviates neonatal necrotizing enterocolitis by repressing the activation of the NLRP3 inflammasome. Gastroenterol. Res Pr. 2022, 6920577 (2022).
Liu, J. et al. Transient receptor potential melastatin 7 promotes vascular adventitial fibroblasts phenotypic transformation and inflammatory reaction induced by mechanical stretching stress via p38 MAPK/JNK pathway. J. Vasc. Res 58, 108–120 (2021).
Park, C. S., Lee, J. Y., Choi, H. Y. & Yune, T. Y. Suppression of transient receptor potential melastatin 7 by carvacrol protects against injured spinal cord by inhibiting blood-spinal cord barrier disruption. J. Neurotrauma 39, 735–749 (2022).
Li, Y. et al. The interaction of transient receptor potential melastatin 7 with macrophages promotes vascular adventitial remodeling in transverse aortic constriction rats. Hypertens. Res 37, 35–42 (2014).
Hao, W. et al. NS8593 inhibits chondrocyte ferroptosis and alleviates cartilage injury in rat adjuvant arthritis through TRPM7 / HO-1 pathway. Int J. Biochem Cell Biol. 174, 106618 (2024).
Li, Q. et al. Transient receptor potential melastatin 7 aggravates necrotizing enterocolitis by promoting an inflammatory response in children. Transl. Pediatr. 11, 2030–2039 (2022).
Carow, B. & Rottenberg, M. E. SOCS3, a major regulator of infection and inflammation. Front Immunol. 5, 58 (2014).
Zhang, H. et al. SOCS3 protects against neonatal necrotizing enterocolitis via suppressing NLRP3 and AIM2 inflammasome activation and p65 nuclear translocation. Mol. Immunol. 122, 21–27 (2020).
Su, Y. et al. Activation of cholinergic anti-inflammatory pathway ameliorates cerebral and cardiac dysfunction after intracerebral hemorrhage through autophagy. Front Immunol. 13, 870174 (2022).
Xia, X.-M. et al. Vagus nerve stimulation as a promising neuroprotection for ischemic stroke via α7nAchR-dependent inactivation of microglial NLRP3 inflammasome. Acta Pharm. Sin. 45, 1349–1365 (2024).
Shen, L. et al. Macrophage α7nAChR alleviates the inflammation of neonatal necrotizing enterocolitis through mTOR/NLRP3/IL-1β pathway. Int Immunopharmacol. 139, 112590 (2024).
Chen, J. et al. MiR-146a-5p mimic inhibits NLRP3 inflammasome downstream inflammatory factors and CLIC4 in neonatal necrotizing enterocolitis. Front Cell Dev. Biol. 8, 594143 (2020).
Yang, X., Li, X., Wu, C. & Zhang, F. Knockdown of PHLDA1 alleviates Necrotizing enterocolitis by inhibiting NLRP3 inflammasome activation and pyroptosis through enhancing Nrf2 signaling. Immunol. Invest 52, 257–269 (2023).
Chen, Z. et al. Bacteroides fragilis alleviates necrotizing enterocolitis through restoring bile acid metabolism balance using bile salt hydrolase and inhibiting FXR-NLRP3 signaling pathway. Gut Microbes 16, 2379566 (2024).
Filler, R. et al. Bovine milk-derived exosomes attenuate NLRP3 inflammasome and NF-κB signaling in the lung during neonatal necrotizing enterocolitis. Pediatr. Surg. Int. 39, 211 (2023).
Yin, Y. et al. Overexpressed FOXO3 improves inflammatory status in mice by affecting NLRP3-mediated cell coronation in necrotizing colitis mice. Biomed. Pharmacother. 125, 109867 (2020).
Acknowledgements
This study was supported by the Health Commission of Guangdong Province (the Medical Science and Technology Research Fund of Guangdong Province) (No. A2024413; No. B2022309), Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515140063), the Dongguan Science and Technology Bureau (the Dongguan Social Development Science and Technology Project) (No. 20231800939962; No. 20231800939932), the Doctoral Research Fund Project of Dongguan Eighth People’s Hospital (No. DBBS2023004).
Author information
Authors and Affiliations
Contributions
Xiuhui Chen and Rui Long drafted the manuscript, made critical revisions, and approved the final submission. Fengdan Xu, Weijun Ye and Ning Li all made critical revisions to the manuscript and approved its final submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Long, R., Xu, F. et al. The role of NLRP3 inflammasome in necrotizing enterocolitis. Pediatr Res 99, 59–67 (2026). https://doi.org/10.1038/s41390-025-04081-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-025-04081-2