Abstract
Background
Respiratory syncytial virus (RSV), adenovirus (ADV), human parainfluenza virus (hPIV), and Mycoplasma pneumoniae (MP) are prevalent pathogens causing acute respiratory infections (ARIs) in children. Prompt and precise detection of these pathogens is essential for early differentiation. This study sought to assess the diagnostic efficacy of a fully automatic real-time fluorescence PCR assay utilizing microfluidic technology (PCR-MT) for the rapid detection of RSV, hPIV, ADV, and MP in children in a hospital setting in Zhejiang, China.
Methods
The study was conducted on 420 children with ARIs from March to December 2022 at our hospital. Throat swab samples were collected and detected for RSV, hPIV, ADV, and MP using both PCR-capillary electrophoresis fragment analysis (PCR-CEFA) and PCR-MT. The results obtained from the PCR-MT method were compared with those from PCR-CEFA.
Results
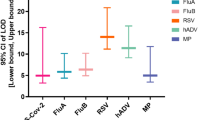
With PCR-CEFA as the gold standard, the sensitivity and specificity of the PCR-MT method were as follows: 94.4% and 100.0% for RSV, 96.0% and 99.1% for hPIV, 100.0% and 98.6% for ADV, and 93.5% and 98.8% for MP, respectively.
Conclusion
The PCR-MT method demonstrates substantial potential for clinical application in the early diagnosis of RSV, hPIV, ADV, and MP in an outpatient setting, offering robust sensitivity and specificity.
Impact
-
Rapid, accurate, and convenient multiple pathogen detection technologies represent a significant area of research in the medical field. The method evaluated in this study enables simultaneous detection of four pathogens on a single chip, covering various subtypes, with results available within half an hour. Although some multi-pathogen detection chips are already commercially available, they may still have limitations such as sensitivity, specificity, and cost. Ongoing technological advancements could make pathogen detection more efficient, accurate, and economical. Continued attention to the development, validation, and optimization of these technologies in clinical practice will better serve patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data presented in this study are available upon reasonable request from the corresponding author.
References
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Rudan, I., Boschi-Pinto, C., Biloglav, Z., Mulholland, K. & Campbell, H. Epidemiology and etiology of childhood pneumonia. Bull. World Health Organ 86, 408–416 (2008).
Li, Z. J. et al. Chinese Centers for Disease Control and Prevention (CDC) Etiology of Respiratory Infection Surveillance Study Team. Etiological and epidemiological features of acute respiratory infections in China. Nat. Commun. 12, 5026 (2021).
Hasan, M. M., Saha, K. K., Yunus, R. M. & Alam, K. Prevalence of acute respiratory infections among children in India: regional inequalities and risk factors. Matern Child Health J. 26, 1594–1602 (2022).
The Lancet Respiratory Medicine. Patterns of respiratory infections after COVID-19. Lancet Respir. Med. 12, 1 (2024).
Maison, N. et al. Old foes following news ways?-Pandemic-related changes in the epidemiology of viral respiratory tract infections. Infection 52, 209–218 (2024).
Zhao, P. et al. Epidemiology of respiratory pathogens in patients with acute respiratory infections during the COVID-19 pandemic and after easing of COVID-19 restrictions. Microbiol. Spectr. 12, e0116124 (2024).
Perez, A. et al. New Vaccine Surveillance Network Collaborators. Respiratory Virus Surveillance Among Children With Acute Respiratory Illnesses - New Vaccine Surveillance Network, United States, 2016-2021. MMWR Morb. Mortal. Wkly Rep. 71, 1253–1259 (2022).
Waites, K. B., Xiao, L., Liu, Y., Balish, M. F. & Atkinson, T. P. Mycoplasma pneumoniae from the Respiratory Tract and Beyond. Clin. Microbiol. Rev. 30, 747–809 (2017).
Zhang, J., Yang, T., Zou, M., Wang, L. & Sai, L. The epidemiological features of respiratory tract infection using the multiplex panels detection during COVID-19 pandemic in Shandong province, China. Sci. Rep. 13, 6319 (2023).
Zhao, M. C. et al. Clinical evaluation of a new single-tube multiplex reverse transcription PCR assay for simultaneous detection of 11 respiratory viruses, Mycoplasma pneumoniae and Chlamydia in hospitalized children with acute respiratory infections. Diagn. Microbiol. Infect. Dis. 88, 115–119 (2017).
Taylor, S. et al. Respiratory viruses and influenza-like illness: epidemiology and outcomes in children aged 6 months to 10 years in a multi-country population sample. J. Infect. 74, 29–41 (2017).
Branche, A. R. & Falsey, A. R. Parainfluenza virus infection. Semin Respir. Crit. Care Med. 37, 538–554 (2016).
Shieh, W. J. Human adenovirus infections in pediatric population - An update on clinico-pathologic correlation. Biomed. J. 45, 38–49 (2022).
Zhan, X. W. et al. Correlation between Mycoplasma pneumoniae drug resistance and clinical characteristics in bronchoalveolar lavage fluid of children with refractory Mycoplasma pneumoniae pneumonia. Ital. J. Pediatr. 48, 190 (2022).
Loaiza, R. A. et al. A molecular perspective for the development of antibodies against the human respiratory syncytial virus. Antivir. Res. 222, 105783 (2024).
Woo, P. C., Chiu, S. S., Seto, W. H. & Peiris, M. Cost-effectiveness of rapid diagnosis of viral respiratory tract infections in pediatric patients. J. Clin. Microbiol. 35, 1579–1581 (1997).
Zheng, X., Quianzon, S., Mu, Y. & Katz, B. Z. Comparison of two new rapid antigen detection assays for respiratory syncytial virus with another assay and shell vial culture. J. Clin. Virol. 31, 130–133 (2004).
Prendergast, C. & Papenburg, J. Rapid antigen-based testing for respiratory syncytial virus: moving diagnostics from bench to bedside? Fut. Microbiol. 8, 435–444 (2013).
Stroparo, E. et al. Adenovirus respiratory infection: significant increase in diagnosis using PCR comparing with antigen detection and culture methods. Rev. Inst. Med. Trop. Sao Paulo 52, 317–321 (2010).
Kuypers, J. et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J. Clin. Microbiol. 44, 2382–2388 (2006).
Buller, R. S. Molecular detection of respiratory viruses. Clin. Lab Med. 33, 439–460 (2013).
Huang, H. S. et al. Multiplex PCR system for the rapid diagnosis of respiratory virus infection: systematic review and meta-analysis. Clin. Microbiol. Infect. 24, 1055–1063 (2018).
Lin, C. Y. et al. Increased detection of viruses in children with respiratory tract infection using PCR. Int. J. Environ. Res. Public Health 17, 564 (2020).
Li, J. et al. Comparison of reverse-transcription qPCR and droplet digital PCR for the detection of SARS-CoV-2 in clinical specimens of hospitalized patients. Diagn. Microbiol. Infect. Dis. 103, 115677 (2022).
Li, H. et al. Simultaneous detection and high-throughput identification of a panel of RNA viruses causing respiratory tract infections. J. Clin. Microbiol. 45, 2105–2109 (2007).
Wang, C. et al. Point-of-care diagnostics for infectious diseases: from methods to devices. Nano Today 37, 101092 (2021).
Huijskens, E. G., Biesmans, R. C., Buiting, A. G., Obihara, C. C. & Rossen, J. W. Diagnostic value of respiratory virus detection in symptomatic children using real-time PCR. Virol. J. 9, 276 (2012).
Altman D. G. Practical Statistics for Medical Research (Hall/CRC, 1991).
Yun, S. G. et al. Comparison of three multiplex PCR assays for detection of respiratory viruses: Anyplex II RV16, AdvanSure RV, and Real-Q RV. J. Clin. Lab Anal. 32, e22230 (2018).
Li, Z. J. et al. Etiological and epidemiological features of acute respiratory infections in China. Nat. Commun. 12, 5026 (2021).
Bont, L. Nosocomial RSV infection control and outbreak management. Paediatr. Respir. Rev. 10, 16–17 (2009).
Wang, X. et al. Respiratory Virus Global Epidemiology Network. Global burden of acute lower respiratory infection associated with human parainfluenza virus in children younger than 5 years for 2018: a systematic review and meta-analysis. Lancet Glob. Health 9, e1077–e1087 (2021).
Lynch, J. P. 3rd & Kajon, A. E. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin. Respir. Crit. Care Med. 37, 586–602 (2016).
Wang, G., Wu, P., Tang, R. & Zhang, W. Global prevalence of resistance to macrolides in Mycoplasma pneumoniae: a systematic review and meta-analysis. J. Antimicrob. Chemother. 77, 2353–2363 (2022).
Acknowledgements
We are grateful to the Department of Clinical Laboratory at The Children’s Hospital of Zhejiang University School of Medicine for supplying the essential laboratory equipment and location required for the collection of experimental data.
Funding
The study was supported by the Natural Science Foundation of Zhejiang Province, China under Grant No.ZCLTGC24H2001 and Medical Health Science and Technology Project of Zhejiang Provincial Health Commission under Grant No.WKJ-ZJ-2534.
Author information
Authors and Affiliations
Contributions
Ying-ying Chen, Formal analysis, Investigation, Writing – original draft, Writing – review and editing. Wen-qing Xiang, Data curation, Formal analysis, Software, Validation, Writing – review and editing. Ya-jun Guo, Investigation, Writing – review and editing. Zheng Shen, Investigation, Writing – review and editing. Wei Li,Writing - review & editing, Supervision, Investigation, Formal analysis, Conceptualization. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study’s design and methodology received approval from the Ethics Committee of the Children’s Hospital at Zhejiang University School of Medicine (Approval No. 2023-IRB-0112-Y-01). Given that the samples used in this research were residual from routine clinical tests, obtaining informed consent was exempted as per the committee’s guidelines. This research adheres to the principles of the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Yy., xiang, Wq., Guo, Yj. et al. Evaluation of a fully automatic nucleic acid amplification system for detecting four respiratory pathogens. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04101-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-04101-1