Abstract
Background
Nephrogenesis occurs during the fetal period; because nephrogenesis does not occur after birth, preterm infants have low nephron numbers. However, whether a reduction in the number of pure nephrons alone affects renal function in conventional animal models remains unclear. Therefore, we aimed to examine whether differences in nephron number alone could lead to differences in future renal function by specifically ablating the nephrons.
Methods
Mice transgenic for Cre recombinase in the nephron progenitor cells were crossed with the locus of X-over P1-diphtheria toxin receptor transgenic mice. Diphtheria toxin was administered on embryonic day 13.5 to suppress fetal nephrogenesis. Various renal function assessments were performed until the maximum age of 1 year. Some mice received a high-salt diet (HSD).
Result
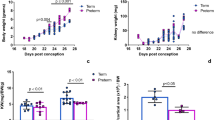
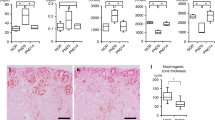
The mouse model showed a glomerular loss of approximately half per cross-sectional area and no or mild renal damage at 1 year of age. Furthermore, HSD induced the early onset and exacerbation of renal impairment.
Conclusion
The new mouse model used in this study showed HSD-induced early onset and exacerbation of renal damage, suggesting that appropriate salt management can prevent the onset and exacerbation of renal impairment in preterm infants known to have low nephron numbers.
Impact
-
We established a new method for generating mice with low nephron numbers without affecting growth.
-
The mouse model did not develop kidney disease but could develop mild kidney disease; however, kidney damage is exacerbated by a high-salt diet.
-
Adequate salt management may prevent the future development of renal damage in infants with low nephron counts when born prematurely.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hinchliffe, S. A. et al. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab. Invest. 64, 777–784 (1991).
Hoogenboom, L. A. et al. Prematurity, perinatal inflammatory stress, and the predisposition to develop chronic kidney disease beyond Oligonephropathy. Pediatr. Nephrol. 36, 1673–1681 (2021).
Murai-Takeda, A. et al. Low birth weight is associated with decline in renal function in Japanese male and female adolescents. Clin. Exp. Nephrol. 23, 1364–1372 (2019).
Crump, C., Sundquist, J., Winkleby, M. A. & Sundquist, K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ 365, l1346 (2019).
Makayes, Y. et al. Increasing Mtorc1 pathway activity or methionine supplementation during pregnancy reverses the negative effect of maternal malnutrition on the developing kidney. J. Am. Soc. Nephrol. 32, 1898–1912 (2021).
Dickinson, H., Walker, D. W., Wintour, E. M. & Moritz, K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R453–R461 (2007).
Moritz, K. M. et al. Prenatal glucocorticoid exposure in the sheep alters renal development in utero: implications for adult renal function and blood pressure control. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R500–R509 (2011).
Schreuder, M. F. et al. Glomerular number and function are influenced by spontaneous and induced low birth weight in rats. J. Am. Soc. Nephrol. 16, 2913–2919 (2005).
Jiang, J. S., Chou, H. C., Yeh, T. F. & Chen, C. M. Neonatal hyperoxia exposure induces kidney fibrosis in rats. Pediatr. Neonatol. 56, 235–241 (2015).
Ali, M. F. et al. Effects of klotho supplementation on hyperoxia-induced renal injury in a rodent model of postnatal nephrogenesis. Pediatr. Res. 88, 565–570 (2020).
Tagawa, M. et al. The reduced number of nephrons with shortening renal tubules in mouse postnatal adverse environment. Pediatr. Res. 93, 1873–1882 (2023).
Fukunaga, S. et al. Optimal route of diphtheria toxin administration to eliminate native nephron progenitor cells in vivo for kidney regeneration. Biochem. Biophys. Res. Commun. 496, 1176–1182 (2018).
Li, Z. et al. 3d Culture Supports Long-Term Expansion of Mouse and Human Nephrogenic Progenitors. Cell Stem Cell 19, 516–529 (2016).
Na, S. Y., Janakiraman, M., Leliavski, A. & Krishnamoorthy, G. High-salt diet Suppresses autoimmune demyelination by regulating the blood-brain barrier permeability. Proc. Natl Acad. Sci. USA118, e2025944118 (2021).
Torres-Pinzon, D. L., Ralph, D. L., Veiras, L. C. & McDonough, A. A. Sex-specific adaptations to high-salt diet preserve electrolyte homeostasis with distinct sodium transporter profiles. Am. J. Physiol. Cell Physiol. 321, C897–C909 (2021).
Truett, G. E. et al. Preparation of Pcr-quality mouse genomic DNA with hot sodium hydroxide and Tris (hotshot). BioTechniques 29, 52 (2000).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Haruhara, K. et al. Podometrics in Japanese living donor kidneys: associations with nephron number, age, and hypertension. J. Am. Soc. Nephrol. 32, 1187–1199 (2021).
Sasaki, T. et al. Bowman capsule volume and related factors in adults with normal renal function. Kidney Int. Rep. 3, 314–320 (2018).
Haruhara, K. et al. Volume ratio of glomerular tufts to bowman capsules and renal outcomes in nephrosclerosis. Am. J. Hypertens. 32, 45–53 (2019).
Denic, A. et al. The substantial loss of nephrons in healthy human kidneys with aging. J. Am. Soc. Nephrol. 28, 313–320 (2017).
Weibel, E. Sterological Method: Practical Methods of Biological Morphometry. (London, Academic Press, 1979).
Kikuchi, H. et al. Signaling mechanisms in renal compensatory hypertrophy revealed by multi-omics. Nat. Commun. 14, 3481 (2023).
Barker, D. J. The origins of the developmental origins theory. J. Intern. Med. 261, 412–417 (2007).
Gazzard, S. E. et al. Nephron deficit and low podocyte density increase risk of albuminuria and glomerulosclerosis in a model of diabetes. Physiol. Rep. 11, e15579 (2023).
Swartling, O. et al. Ckd progression and mortality among men and women: A nationwide study in Sweden. Am. J. Kidney Dis. 78, 190–199.e191 (2021).
Tsai, W. C. et al. Risk factors for development and progression of chronic kidney disease: A systematic review and exploratory meta-analysis. Medicine95, e3013 (2016).
Zhao, J. V. & Schooling, C. M. Sex-specific associations of sex hormone binding globulin with Ckd and kidney function: A univariable and multivariable Mendelian randomization study in the UK Biobank. J. Am. Soc. Nephrol. 32, 686–694 (2021).
Hannan, M. et al. Risk factors for Ckd progression: overview of findings from the cric study. Clin. J. Am. Soc. Nephrol. 16, 648–659 (2021).
Ajalbert, G. et al. Elevation of arginase-Ii in podocytes contributes to age-associated albuminuria in male mice. Int. J. Mol. Sci. 24, 11228 (2023).
Acknowledgements
We would like to thank T. Tamatsukuri, H. Hayashi, M. Ishida, S. Nishimura, S. Kawagoe, and T. Hayakawa for technical assistance with this study. We also thank Y. Takemura of Core Research Facilities of Jikei University School of Medicine for technical assistance with electron microscopy analysis and Editage (www.editage.jp) for English language editing. This work was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (KAKENHI) (grant numbers 20K17258 and 23K07725) and the Kidney Research Initiative-Japan (KRI-J: Japan Kidney Association and Nippon Boehringer Ingelheim Joint Research Project).
Author information
Authors and Affiliations
Contributions
Y.I., Kei Matsumoto, K.H., and D.H. made substantial contributions to the study conception and design and data acquisition, analysis, and interpretation. Kenji Matsui, Y.K., Keita Morimoto, N.K., Shutaro Yamamoto, T.F., S.F., and Shuichiro Yamanaka contributed to the methodology and data collection. K.O., E.K., and T.Y. were major contributors to the review and editing of the manuscript. All the authors discussed the results and reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Yuka Inage, Kei Matsumoto, Kenji Matsui, Yoshitaka Kinoshita, Keita Morimoto, Nagisa Koda, Shutaro Yamamoto, Toshinari Fujimoto, Shohei Fukunaga, Kotaro Haruhara, Shuichiro Yamanaka, Daishi Hirano, Kimihiko Oishi, and Takashi Yokoo declare that they have no conflicts of interest or financial conflicts to disclose. Eiji Kobayashi is the Chief Information Officer of Kobayashi Regeneration Research Institute, LLC. The funders had no role in the study design, collection, analyses, or interpretation of data, writing of the manuscript, or decision to publish the results.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Inage, Y., Matsumoto, K., Haruhara, K. et al. A novel mouse model for investigating the long-term impact of reduced nephron numbers on renal function and salt sensitivity. Pediatr Res 98, 1941–1949 (2025). https://doi.org/10.1038/s41390-025-04123-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-025-04123-9