Abstract
Background
As many jurisdictions move towards seasonal COVID-19 vaccines, there remains insufficient data on optimal vaccination strategies for children with immune-mediated inflammatory diseases (IMID) treated with immunomodulatory therapies.
Methods
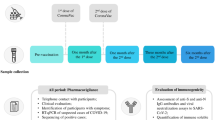
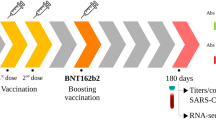
A prospective observational cohort study was performed, with clinical data and biosamples longitudinally collected to determine the effect of immunomodulatory therapies on the antibody response to four COVID-19 vaccine doses. Antibodies against the SARS-CoV-2 spike, receptor-binding domain, and nucleocapsid proteins were measured.
Results
Following doses two and three, antibody responses were lowest in participants treated with biologic or targeted synthetic disease-modifying anti-rheumatic drugs (b/tsDMARDs), of which TNF inhibitors were the most common. Rates of antibody decay were similar between treatment groups, but weaker initial responses in the b/tsDMARD group led to antibody levels dipping to low values within four months of vaccination. The fourth vaccine dose significantly boosted antibody levels in the b/tsDMARD group, resulting in a sustained response. Among additional samples collected from children with hybrid immunity (i.e., vaccinated and prior SARS-CoV-2 infection), no differences in antibody levels were found between participants who were or were not treated with b/tsDMARDs.
Conclusions
These data support providers in counselling families regarding the importance of annual COVID-19 booster vaccines in children with IMID.
Impact
-
There is conflicting evidence on the effect of immunosuppressive treatments on the antibody response to vaccination in paediatric populations.
-
Long-term follow-up of children treated with various immunosuppressive therapies as they received booster COVID-19 vaccines demonstrated that antibody responses to the second and third vaccine dose were impaired in children treated with biologics (mainly TNF inhibitors).
-
Deficits in the antibody response were largely corrected by a fourth vaccine dose or infection with SARS-CoV-2.
-
Children treated with biologic therapies require a full suite of COVID-19 vaccines and should be strongly encouraged to receive seasonal COVID-19 vaccine boosters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Watanabe, A. et al. Assessment of efficacy and safety of mRNA COVID-19 vaccines in children aged 5 to 11 years: a systematic review and meta-analysis. JAMA Pediatr.177, 384–394 (2023).
Government of Canada. Covid-19 Vaccination: Vaccination Coverage, https://health-infobase.canada.ca/covid-19/vaccination-coverage/#a5 (2024).
Government of Canada. in Canadian Immunization Guide (2024).
Panagiotakopoulos, L. Use of Covid-19 Vaccines for Persons Aged≥ 6 Months: Recommendations of the Advisory Committee on Immunization Practices—United States, 2024–2025. Morbid. Mortal Wkly Rep. 73 (2024).
Martini, A. et al. Juvenile idiopathic arthritis. Nat. Rev. Dis. Prim. 8, 1–18 (2022).
Ravelli, A. et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann. Rheum. Dis. 77, 819–828 (2018).
Giancane,G. & Ruperto, N.Paediatric Rheumatology International Trials OrganisationTreatment of juvenile idiopathic arthritis: What’s new?Curr. Opin. Rheumatol.31,428–435 (2019).
Sullivan, K. E. Pathogenesis of pediatric rheumatologic diseases. Pediatr. Clin. 65, 639–655 (2018).
Sadeghi, P., Pezeshki, P. S. & Rezaei, N. Coronavirus Disease 2019 (COVID-19) in Pediatric Patients with Autoimmune Disorders. Eur. J. Pediatr., 1–22 (2023).
Xie, Y., Liu, Y. & Liu, Y. The flare of rheumatic disease after SARS-CoV-2 Vaccination: A Review. Front. Immunol. 13, 919979 (2022).
Akgün, Ö. et al. Humoral response and safety of BNT162b2 mRNA vaccine in children with rheumatic diseases. Rheumatol. Oxf. Engl. 61, keac140 (2022).
Kashiwagi, K. et al. Impact of Anti-Tnfα treatment on the humoral response to the BNT162b2 mRNA COVID-19 vaccine in pediatric inflammatory bowel disease patients. Vaccines 10, 1618 (2022).
Shire, Z. J. et al. Antibody response to the BNT162b2 SARS-CoV-2 vaccine in paediatric patients with inflammatory bowel disease treated with anti-TNF therapy. Gut 71, 1922–1924 (2022).
Dimopoulou, D., Vartzelis, G., Dasoula, F., Tsolia, M. & Maritsi, D. Immunogenicity of the COVID-19 mRNA vaccine in adolescents with juvenile idiopathic arthritis on treatment with Tnf inhibitors. Ann. Rheum. Dis. 81, 592–593 (2022).
Dimopoulou, D., Tsolia, M. N., Spyridis, N. & Maritsi, D. N. Immunogenicity 6 months post Covid-19 Mrna vaccination among adolescents with juvenile idiopathic arthritis on treatment with TNF inhibitors. Rheumatol. Oxf. Engl. 62, keac352 (2022).
Gicchino, M. F., Abbate, F. G., Amodio, A., Giudice, E. M. D. & Olivieri, A. N. Preliminary observations on the immunogenicity and safety of vaccines to prevent Covid-19 in patients with juvenile idiopathic arthritis. Acta Paediatr.111, 2359–2361 (2022).
Yeo, J. G. et al. Robust neutralizing antibody response to SARS-CoV-2 mRNA vaccination in adolescents and young adults with childhood-onset rheumatic diseases. Rheumatol. Oxf. Engl. 61, keac105 (2022).
Yeo, J. G. et al. COVID-19 mRNA vaccine immunogenicity decay and breakthrough illness in adolescents and young adults with childhood-onset rheumatic diseases. Rheumatology (2023).
Heshin-Bekenstein, M. et al. Safety and Immunogenicity of BNT162b2 mRNA COVID-19 vaccine in adolescents with rheumatic diseases treated with immunomodulatory medications. Rheumatol. Oxf. Engl. 61, keac103 (2022).
Heshin-Bekenstein, M. et al. Safety and immunogenicity following the second and third doses of the BNT162b2 mRNA COVID-19 vaccine in adolescents with juvenile-onset autoimmune inflammatory rheumatic diseases: a prospective multicentre study. Vaccines 11, 819 (2023).
Cotugno, N. et al. Evaluation of safety and immunogenicity of BNT162b2 mRNA COVID-19 vaccine in Ibd Pediatric Population with Distinct Immune Suppressive Regimens. Vaccines 10, 1109 (2022).
Kamei, K. et al. Immunogenicity and safety of SARS-CoV-2 vaccine with immunosuppressive agents. Pediatr. Int. 64, e15331 (2022).
Udaondo, C. et al. Humoral and cellular immune response to mRNA SARS-CoV-2 BNT162b2 vaccine in adolescents with rheumatic diseases. Pediatr. Rheumatol. 20, 64 (2022).
Hanberg, J. S. et al. Effectiveness of a fourth dose of COVID-19 mRNA vaccine in patients with systemic autoimmune rheumatic diseases using disease-modifying antirheumatic drugs: an emulated target trial. Lancet Rheumatol. 6, e21–e30 (2024).
Bjørlykke, K. H. et al. Four Sars-Cov-2 vaccine doses or hybrid immunity in patients on immunosuppressive therapies: A Norwegian Cohort Study. Lancet Rheumatol. 5, e36–e46 (2023).
Simon, A. K., Hollander, G. A. & McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 282, 20143085 (2015).
Howard-Jones, A. R. et al. Covid-19 in Children. II: Pathogenesis, disease spectrum and management. J. Paediatr. Child Health 58, 46–53 (2022).
Wahezi, D. M. et al. American College of Rheumatology Guidance for the Management of Pediatric Rheumatic Disease during the COVID-19 Pandemic: Version 2. Arthritis Rheumatol. 73, e46–e59 (2021).
Landewé, R. B. et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: The November 2021 Update. Ann. Rheum. Dis. 81, 1628–1639 (2022).
Murphy, T. J. et al. The evolution of Sars-Cov-2 Seroprevalence in Canada: A Time-Series Study, 2020–2023. CMAJ 195, E1030–E1037 (2023).
Colwill, K. et al. A scalable serology solution for profiling humoral immune responses to SARS-CoV-2 infection and vaccination. Clin. Transl. Immunol. 11, e1380 (2022).
Hyrich, K. L. et al. Disease activity and disability in children with juvenile idiopathic arthritis one year following presentation to paediatric rheumatology. results from the childhood arthritis prospective study. Rheumatology 49, 116–122 (2010).
Salmon, D. A. et al. Association between Guillain-Barré Syndrome and Influenza a (H1n1) 2009 Monovalent Inactivated Vaccines in the USA: A Meta-Analysis. Lancet 381, 1461–1468 (2013).
Colwill, K. et al. in COVID-19 Immunity Task Force (2023).
Powell, A. A., Dowell, A. C., Moss, P. & Ladhani, S. N. Current State of Covid-19 in Children: 4 Years On. J. Infect., 106134 (2024).
Anolik, J. H. et al. Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits Memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J. Immunol. 180, 688–692 (2008).
Kobie, J. J. et al. Decreased Influenza-Specific B cell responses in rheumatoid arthritis patients treated with anti-tumor necrosis factor. Arthritis Res. Ther. 13, 1–12 (2011).
Cassese, G. et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 171, 1684–1690 (2003).
Swart, J. et al. Pharmacovigilance in juvenile idiopathic arthritis patients treated with biologic or synthetic drugs: combined data of more than 15,000 patients from PharmChild and national registries. Arthritis Res. Ther. 20, 1–11 (2018).
Becker, I. & Horneff, G. Risk of serious infection in juvenile idiopathic arthritis patients associated with tumor necrosis factor inhibitors and disease activity in the German biologics in Pediatric Rheumatology Registry. Arthritis Care Res. 69, 552–560 (2017).
Maritsi, D. N., Kopsidas, I., Vartzelis, G., Spyridis, N. & Tsolia, M. N. Long-term preservation of measles and rubella-specific IgG antibodies in children with enthesitis-related arthritis on anti-tnfα treatment: a prospective controlled study. Rheumatology 58, 1686–1688 (2019).
Jansen, M. H. et al. Efficacy, immunogenicity and safety of vaccination in pediatric patients with autoimmune inflammatory rheumatic diseases (PEDAIIRD): A systematic literature review for the 2021 Update of the EULAR/Pres Recommendations. Front. Pediatr. 10 (2022).
Goldblatt, D., Alter, G., Crotty, S. & Plotkin, S. A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol. Rev. 310, 6–26 (2022).
Morgan, G. et al. Characterizing the Sars-Cov-2 antibody response and associations with patient factors: serological profiling of participants enrolled in the Gencov Study. Clin. Biochem. 135, 110859 (2025).
Acknowledgements
We thank Geneviève Mailhot, Melanie Delgado-Brand, Tulunay Tursun, Martina Tersigni, Roya M. Dayam, and Freda Qi for their role in SARS-CoV-2 serology analysis, which was conducted using robotic equipment in the Network Biology Collaborative Centre High-Throughput Screening Facility (RRID: SCR_025390), a facility supported by the Canada Foundation for Innovation and the Ontario Government. Antigens, protein standards, and secondary antibodies for serum ELISA were kindly provided by the Pandemic Response Challenge Program of the National Research Council of Canada (Dr. Yves Durocher).
Funding
This project was supported by funding from the Government of Canada, through the COVID-19 Immunity Task Force. JRS was supported by a fellowship from the Canadian Immunization Research Network. RSMY was supported by the Hak Ming and Deborah Chiu Chair in Paediatric Translational Research from the Hospital for Sick Children, University of Toronto. ACG is the Canada Research Chair in Functional Proteomics and the Sinai 100 Louis Siminovitch Chair in Research. SMB was supported by the Cenovus Energy Chair and the ACHF Chair for Pediatric Research from the Alberta Children’s Hospital Foundation and the University of Calgary. EIB holds the Northbridge Financial Corporation Chair in Inflammatory Bowel Disease, a joint Hospital-University Chair between the University of Toronto, The Hospital for Sick Children, and the SickKids Foundation. LH holds a Canada Research Chair in Genetics of Rare Systemic Inflammatory Diseases.
Author information
Authors and Affiliations
Consortia
Contributions
R.S.M.Y. oversaw all aspects of the study. R.S.M.Y., S.B. and S.B. conceptualized the study and acquired funding. A.X. and T.D. were responsible in project administration, data collection, and sample processing. The members of the SUCCEED Kids study team recruited participants and collected data. A.C.G. and K.C. performed and oversaw the serology testing. J.R.S., F.C. and A.X. curated data. JRS performed formal data analysis, created figures, and drafted the manuscript. All authors reviewed the manuscript and provided editorial contributions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Written informed consent/assent was obtained from all participants and/or their substitute decision-makers.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shapiro, J.R., Choi, F., Xu, A. et al. Immunogenicity of COVID-19 booster vaccines in children receiving immunosuppressive medications. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04174-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04174-y