Abstract
Background
Tertiary lymphoid structures (TLSs) have emerged as critical regulators of antitumor immunity and prognostic indicators in various malignancies. However, the distribution patterns and prognostic significance of TLSs in hepatoblastoma (HB) remain poorly understood. This study aimed to investigate the presence, distribution, and prognostic value of TLSs in HB patients following neoadjuvant chemotherapy and to explore the underlying mechanisms linking TLSs to the tumor immune microenvironment.
Methods
A total of 112 HB patients who underwent neoadjuvant chemotherapy and surgical resection at Shandong Provincial Hospital between 2015 and 2024 were retrospectively enrolled. The presence of TLSs was evaluated using hematoxylin and eosin (H&E) staining, and patients were classified into TLS-positive and TLS-negative groups. Univariate and multivariate Cox regression analyses were performed to identify independent prognostic factors for overall survival (OS). In addition, transcriptome data from the GEO database (GSE133039) were analyzed to construct a TLS gene signature score and explore immune-related mechanisms associated with TLS presence.
Results
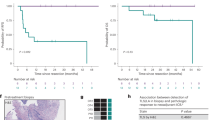
TLSs were identified in 45 out of 112 hepatoblastoma patients (40.2%). Kaplan-Meier survival analysis demonstrated that TLS-positive patients had significantly longer overall survival (OS) compared to TLS-negative patients (p = 0.0017). Multivariate Cox regression analysis further confirmed the presence of TLSs as an independent favorable prognostic factor (HR = 0.061, p = 0.027). In contrast, advanced PRETEXT stage (III/IV), vascular invasion, and distant metastasis were identified as independent adverse prognostic factors, indicating that patients diagnosed at later stages tended to have a worse prognosis. Transcriptomic analysis revealed that TLS-positive tumors exhibited higher expression of antigen presentation and immune activation-related genes (e.g., HLA-DQA1, HLA-DQB1, SLAMF7), along with enriched infiltration of B cells, CD8+ T cells, and NK cells, suggesting a more active antitumor immune microenvironment.
Conclusion
The presence of TLSs is significantly associated with favorable prognosis in HB patients and may contribute to enhanced antitumor immunity by recruiting and activating cytotoxic immune cells. TLSs represent a promising prognostic biomarker and potential immunotherapeutic target for HB patients.
Impact
-
Tertiary lymphoid structures (TLSs) serve as a promising prognostic biomarker in hepatoblastoma (HB).
-
Our study demonstrates that TLS-positive patients exhibit significantly prolonged overall survival.
-
TLSs contribute to the tumor immune microenvironment by recruiting cytotoxic immune cells.
-
These findings provide new insights into TLSs as a potential immunotherapeutic target for HB patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Ayllon Teran, D. et al. Efficacy of neoadjuvant therapy and surgical rescue for locally advanced hepatoblastomas: 10 year single-center experience and literature review. World J. Gastroenterol. 20, 10137–11043 (2014).
Waters, A. M., Mathis, M. S., Beierle, E. A. & Russell, R. T. A synopsis of pediatric patients with hepatoblastoma and Wilms tumor: NSQIP-P 2012–2016. J. Surg. Res. 244, 338–342 (2019).
Hadzic, N. & Finegold, M. J. Liver neoplasia in children. Clin. Liver Dis. 15, 443–462 (2011).
Finegold, M. J. et al. Liver tumors: pediatric population. Liver Transpl. 14, 1545–1556 (2008).
Allan, B. J. et al. Predictors of survival and incidence of hepatoblastoma in the paediatric population. HPB15, 741–746 (2013).
Herzog, C. E., Andrassy, R. J. & Eftekhari, F. Childhood cancers: hepatoblastoma. Oncologist 5, 445–453 (2000).
Schnater, J. M. et al. Surgical view of the treatment of patients with hepatoblastoma: results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group. Cancer 94, 1111–1120 (2002).
Shukla, P. J. et al. Hepatoblastoma: a single institutional experience of 18 cases. Pediatr. Surg. Int. 24, 799–802 (2008).
von Schweinitz, D. et al. Efficiency and toxicity of ifosfamide, cisplatin and doxorubicin in the treatment of childhood hepatoblastoma. Study Committee of the Cooperative Paediatric Liver Tumour Study HB89 of the German Society for Paediatric Oncology and Haematology. Eur. J. Cancer 33, 1243–1249 (1997).
Tsay, P. K. et al. Treatment outcomes for hepatoblastoma: experience of 35 cases at a single institution. J. Formos. Med. Assoc. 110, 322–325 (2011).
Sautès-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019).
Zhao, Y. Y. et al. Density of tertiary lymphoid structures predicts clinical outcome in breast cancer brain metastasis. J. Immunother. Cancer 12, e009232 (2024).
Ren, F. et al. Tertiary lymphoid structures in lung adenocarcinoma: characteristics and related factors. Cancer Med. 11, 2969–2977 (2022).
Zhang, C. et al. Localization and density of tertiary lymphoid structures associate with molecular subtype and clinical outcome in colorectal cancer liver metastases. J. Immunother. Cancer 11, e006425 (2023).
Dushyanthen, S. et al. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 13, 202 (2015).
Webb, J. R. et al. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin. Cancer Res. 20, 434–444 (2014).
Zhang, L. et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin. Cancer Res. 21, 2278–2288 (2015).
Groen-van Schooten, T. S. et al. Mapping the complexity and diversity of tertiary lymphoid structures in primary and peritoneal metastatic gastric cancer. J. Immunother. Cancer 12, e009243 (2024).
Finkin, S. et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat. Immunol. 16, 1235–1244 (2015).
Dieu-Nosjean, M. C. et al. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol. Rev. 271, 260–275 (2016).
Ma, L. et al. Clinicopathological and prognostic value of tertiary lymphoid structures in lung cancer: a meta-analysis. Clin. Transl. Oncol. 27, 1092–1104 (2025).
Xu, W. et al. Heterogeneity in tertiary lymphoid structures predicts distinct prognosis and immune microenvironment characterizations of clear cell renal cell carcinoma. J. Immunother. Cancer 11, e006667 (2023).
Xiao, Y. & Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharm. Ther. 221, 107753 (2021).
Denton, A. E., Roberts, E. W. & Fearon, D. T. Stromal cells in the tumor microenvironment. Adv. Exp. Med Biol. 1060, 99–114 (2018).
Arneth, B. Tumor microenvironment. Medicina (Kaunas, Lithuania) 56, 15–30 (2019).
Aoki, T. et al. Single-cell transcriptome analysis reveals disease-defining T-cell subsets in the tumor microenvironment of classic Hodgkin lymphoma. Cancer Discov. 10, 406–421 (2020).
Hinshaw, D. C. & Shevde, L. A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 79, 4557–4566 (2019).
Teillaud, J. L. & Dieu-Nosjean, M. C. Tertiary lymphoid structures: an anti-tumor school for adaptive immune cells and an antibody factory to fight cancer?. Front. Immunol. 8, 830 (2017).
Zhu, W. et al. A high density of tertiary lymphoid structure B cells in lung tumors is associated with increased CD4(+) T cell receptor repertoire clonality. Oncoimmunology 4, e1051922 (2015).
Shi, J. Y. et al. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin. Cancer Res 19, 5994–6005 (2013).
Laumont, C. M. et al. Tumour-infiltrating B cells: immunological mechanisms, clinical impact and therapeutic opportunities. Nat. Rev. Cancer 22, 414–430 (2022).
Shen, P. & Fillatreau, S. Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol. 15, 441–451 (2015).
Rodriguez, A. B. et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors via cancer-associated fibroblasts. Cell Rep. 36, 109422 (2021).
Böttcher, J. P. et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037.e14 (2018).
Barry, K. C. et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 24, 1178–1191 (2018).
Palucka, A. K. & Coussens, L. M. The basis of oncoimmunology. Cell 164, 1233–1247 (2016).
An, Y. et al. Tertiary lymphoid structure patterns aid in identification of tumor microenvironment infiltration and selection of therapeutic agents in bladder cancer. Front. Immunol. 13, 1049884 (2022).
Dieu-Nosjean, M. C. et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 26, 4410–4417 (2008).
Chaudhary, P., Srivastava, P. & Manna, P. P. Effector functions of dendritic cells in cancer: role of cytotoxicity and growth inhibition. Front Biosci.29, 293 (2024).
Noubade, R., Majri-Morrison, S. & Tarbell, K. V. Beyond cDC1: emerging roles of DC crosstalk in cancer immunity. Front. Immunol. 10, 1014 (2019).
Weng, Y. et al. The impact of tertiary lymphoid structures on tumor prognosis and the immune microenvironment in non-small cell lung cancer. Sci. Rep. 14, 16246 (2024).
Ghasemi, F. et al. High MHC-II expression in Epstein-Barr virus-associated gastric cancers suggests that tumor cells serve an important role in antigen presentation. Sci. Rep. 10, 14786 (2020).
Rimsza, L. M. et al. Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: a follow-up study from the Leukemia and Lymphoma Molecular Profiling Project. Blood 103, 4251–4258 (2004).
Leite, F. A. et al. Low expression of HLA-DRA, HLA-DPA1, and HLA-DPB1 is associated with poor prognosis in pediatric adrenocortical tumors (ACT). Pediatr. Blood Cancer 61, 1940–1948 (2014).
Lyu, L. et al. Overexpressed pseudogene HLA-DPB2 promotes tumor immune infiltrates by regulating HLA-DPB1 and indicates a better prognosis in breast cancer. Front Oncol. 10, 1245 (2020).
Zhong, M. C. et al. SLAM family receptors control pro-survival effectors in germinal center B cells to promote humoral immunity. J. Exp. Med. 218, e20200756 (2021).
Hasim, M. S. et al. When killers become thieves: trogocytosed PD-1 inhibits NK cells in cancer. Sci. Adv. 8, eabj3286 (2022).
Veillette, A., Dong, Z. & Latour, S. Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity 27, 698–710 (2007).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Dieu-Nosjean, M. C. Tumor-associated tertiary lymphoid structures: a cancer biomarker and a target for next-generation immunotherapy. Adv. Exp. Med. Biol. 1329, 51–68 (2021).
Trüb, M. & Zippelius, A. Tertiary lymphoid structures as a predictive biomarker of response to cancer immunotherapies. Front. Immunol. 12, 674565 (2021).
Ladányi, A. et al. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol. Immunother. 56, 1459–1469 (2007).
McMullen, T. P. et al. Survival in rectal cancer is predicted by T cell infiltration of tumour-associated lymphoid nodules. Clin. Exp. Immunol. 161, 81–88 (2010).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Funding
This study was supported by the Shandong Provincial Natural Science Foundation (No. ZR2024QH349) and the Incubation Foundation of Shandong Provincial Hospital (No. 2023FY051).
Author information
Authors and Affiliations
Contributions
S.R.W. wrote the article, L.Z.P. and Z.Y.S. collected case data, G.H.J. and H.Y.J. were responsible for designing the research plan, Y.F.J., J.Q.R. and J.Y.P. conducted statistical analysis, Z.S.Z. and Z.Q.X. assisted in patient coordination, N.Z.Y. and J.L. provided guidance for the article writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. This retrospective study was approved by the Biomedical Ethics Committee of Shandong Prov incial Hospital (Approval No. SWYX2025-217). The need for informed consent was waived.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, R., Liu, Z., Zhang, Y. et al. Density of tertiary lymphoid structures predict clinical outcome in hepatoblastoma. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04210-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-04210-x