Abstract
Background
Febrile infants ≤90 days are at high risk of serious bacterial infections, but prompt differentiation between viral and bacterial infections remains a challenge. Host gene expression signatures, particularly those involving the type I interferon (IFN) pathway, show promise as diagnostic tools. This study evaluates the ability of IFN signature to differentiate bacterial from viral infections in this population.
Methods
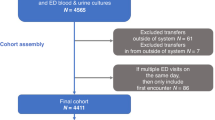
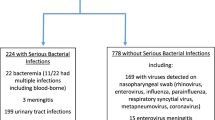
This is a prospective, single-center study. Infants ≤90 days were enrolled. All patients underwent complete blood count, CRP, PCT, and IFN signature, assessed by analyzing the expression of six interferon-stimulated genes. Based on routine investigations, patients were divided into bacterial (BI, n = 8) and non-bacterial infection (non-BI, n = 39) and healthy controls (HC, n = 3). Regression analysis was used to determine the variable’s predictive correlation.
Results
Forty-seven febrile infants were enrolled. IFN signature resulted significantly elevated in non-BI compared to BI (p < 0.001) and HC (p = 0.008), even within 12 h of fever onset. IFN demonstrated the highest AUC for predicting BI. Combining IFN with CRP or PCT yielded the most robust predictive models (AUC = 0.99 and 0.98).
Conclusions
IFN signature resulted as a promising, reliable predictor of BI versus non-BI in febrile infants. This novel biomarker, combined with others, could improve the management of febrile infants reducing unnecessary antibiotics and hospitalizations.
Impact
-
This is the first study to focus on the IFN signature in febrile infants under 90 days old, adding novel insights into the potential of host immune responses as diagnostic biomarkers.
-
This study demonstrates that the peripheral blood interferon signature, specifically the expression of six IFN-stimulated genes, can distinguish bacterial from non-bacterial infections in febrile infants aged 90 days or younger in the very first hours of fever.
-
The IFN score, when combined with traditional inflammatory markers, offers high sensitivity and specificity, making it a potential diagnostic tool for optimizing treatment and reducing unnecessary interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Pantell, R. H. et al. Evaluation and management of well-appearing febrile infants 8 to 60 days old. Pediatrics 148, e2021052228 (2021).
Biondi, E. A. et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life: a systematic review and meta-analysis. JAMA Netw. Open 2, e190874 (2019).
Powell, E. C. et al. Epidemiology of bacteremia in febrile infants aged 60 days and younger. Ann. Emerg. Med. 71, 211–216 (2018).
Aronson, P. L. et al. Variation in care of the febrile young infant, 90 days in us pediatric emergency departments. Pediatrics 134, 667–677 (2014).
Gomez, B. et al. Validation of the “step-by-step” approach in the management of young febrile infants. Pediatrics 138, e20154381 (2016).
Bonilla, L. et al. Prevalence of bacterial infection in febrile infant 61-90 days old compared with younger infants. Pediatr. Infect. Dis. J. 38, 1163–1167 (2019).
Velasco, R. et al. Performance of febrile infant algorithms by duration of fever. Pediatrics 153, e2023064342 (2024).
Bell, B. G., Schellevis, F., Stobberingh, E., Goossens, H. & Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 14, 13 (2014).
Coyle, C., Brock, G., Wallihan, R. & Leonard, J. C. Cost Analysis of emergency department criteria for evaluation of febrile infants ages 29 to 90 days. J. Pediatr. 231, 94.e2–101.e2 (2021).
Jaskiewicz, J. A. et al. Febrile infants at low risk for serious bacterial infection–an appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics 94, 390–396 (1994).
Kuppermann, N. et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 173, 342 (2019).
Aronson, P. L. et al. A prediction model to identify febrile infants ≤60 days at low risk of invasive bacterial infection. Pediatrics 144, e20183604 (2019).
Gomez, B. et al. Diagnostic value of procalcitonin in well-appearing young febrile infants. Pediatrics 130, 815–822 (2012).
Tsao, Y.-T. et al. Differential markers of bacterial and viral infections in children for point-of-care testing. Trends Mol. Med. 26, 1118–1132 (2020).
Gómez-Carballa, A. et al. A qPCR expression assay of IFI44L gene differentiates viral from bacterial infections in febrile children. Sci. Rep. 9, 11780 (2019).
Pennisi, I. et al. Translation of a host blood RNA signature distinguishing bacterial from viral infection into a platform suitable for development as a point-of-care test. JAMA Pediatr. 175, 417 (2021).
Tsalik, E. L. et al. Discriminating bacterial and viral infection using a rapid host gene expression test. Crit. Care Med. 49, 1651–1663 (2021).
Zaas, A. K. et al. Gene expression signatures diagnose influenza and other symptomatic respiratory viral infections in humans. Cell Host Microbe 6, 207–217 (2009).
Atallah, J. & Mansour, M. K. Implications of using host response-based molecular diagnostics on the management of bacterial and viral infections: a review. Front. Med. 9, 805107 (2022).
Buonsenso, D., Sodero, G. & Valentini, P. Transcript host-RNA signatures to discriminate bacterial and viral infections in febrile children. Pediatr. Res. 91, 454–463 (2022).
Mahajan, P. et al. Association of RNA biosignatures with bacterial infections in febrile infants aged 60 days or younger. JAMA 316, 846 (2016).
Kaforou, M., Herberg, J. A., Wright, V. J., Coin, L. J. M. & Levin, M. Diagnosis of bacterial infection using a 2-transcript host RNA signature in febrile infants 60 days or younger. JAMA 317, 1577 (2017).
Barral-Arca, R., Pardo-Seco, J., Martinón-Torres, F. & Salas, A. A 2-transcript host cell signature distinguishes viral from bacterial diarrhea and it is influenced by the severity of symptoms. Sci. Rep. 8, 8043 (2018).
Stetson, D. B. & Medzhitov, R. Type I interferons in host defense. Immunity 25, 373–381 (2006).
Trouillet-Assant, S. et al. Type I interferon in children with viral or bacterial infections. Clin. Chem. 66, 802–808 (2020).
Herberg, J. A. et al. Diagnostic test accuracy of a 2-transcript host RNA signature for discriminating bacterial vs viral infection in febrile children. JAMA 316, 835 (2016).
Tian, S. et al. FAM89A and IFI44L for distinguishing between viral and bacterial infections in children with febrile illness. Pediatr. Investig. 5, 195–202 (2021).
Chawla, D. G. et al. Benchmarking transcriptional host response signatures for infection diagnosis. Cell Syst. 13, 974.e7–988.e7 (2022).
Bodkin, N. et al. Systematic comparison of published host gene expression signatures for bacterial/viral discrimination. Genome Med. 14, 18 (2022).
McClain, M. T. et al. A blood-based host gene expression assay for early detection of respiratory viral infection: an index-cluster prospective cohort study. Lancet Infect. Dis. 21, 396–404 (2021).
Tesser, A. et al. Type I interferon signature: a quantitative standardized method for clinical application. Clin. Exp. Immunol. 219, uxaf018 (2025).
Garcia, S. et al. Is 15 days an appropriate cut-off age for considering serious bacterial infection in the management of febrile infants? Pediatr. Infect. Dis. J. 31, 455–458 (2012).
Gomez, B., Diaz, H., Carro, A., Benito, J. & Mintegi, S. Performance of blood biomarkers to rule out invasive bacterial infection in febrile infants under 21 days old. Arch. Dis. Child. 104, 547–551 (2019).
Bellini, T. et al. Repeated inflammatory markers may be useful for assessing febrile infants aged 29–90 days during early hospital surveillance. Acta Paediatr. 112, 1056–1057 (2023).
Author information
Authors and Affiliations
Contributions
E.F., T.B., S.V., and R.P. conceived the idea; E.F., G.V., M.B., F.S., and E.P. collected the samples; P.B. performed the interferon signature analysis; E.F. and T.B. performed the experiments and analyzed the data; E.F. and T.B. prepared the first draft. S.V. and E.P. revised the manuscript. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Approval for this study was granted by the local Ethics Committee (CET - Liguria:262/2024 - DB id 13963). Informed consent was obtained from the parents of all patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fueri, E., Bocca, P., Villa, G. et al. Early diagnosis of serious bacterial infection in febrile infants using type I interferon signature. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04229-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-04229-0