Abstract
Background
Feeding difficulties significantly impact neonatal well-being. This study explores the clinical characteristics, etiologies, and diagnostic practices for full-term neonates with feeding difficulties in neonatal intensive care units (NICUs).
Methods
This retrospective cohort study recruited full-term infants admitted to NICUs participating in the China Neonatal Genome Project from March 2017 to December 2021, diagnosed with feeding difficulties persisting >72 h.
Results
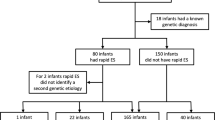
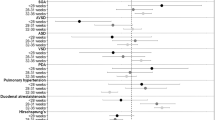
Among 220 patients, the most common symptoms were poor sucking (39.5%), vomiting (22.3%), and dysphagia (14.1%). High-yield diagnostic modalities included genetic tests (83/220, 37.3%), brain imaging (70/145, 48.3%), laryngoscopy (47/54, 87.0%), and muscle biopsy (12/28, 42.9%). A definitive etiology was identified through clinical evaluation in 100 cases (45.5%), 48 of which (21.8%) were subsequently confirmed by genetic testing. In an additional 35 cases (15.9%), genetic results contributed to diagnostic clarification or revision. Compared to neonates without genetic disorders, the 83 patients with genetic disorders were more likely to have persistent feeding difficulties, reduced muscle tone, craniofacial deformities, urinary and reproductive system malformations, and a need for invasive respiratory support (P < 0.05 for all).
Conclusion
Identifiable etiologies were found in over 60% cases, with genetic disorders representing a significant subset. Selective genetic testing and targeted diagnostic strategies are essential for managing feeding difficulties in this vulnerable population.
Impact
Feeding difficulties in full-term neonates admitted to NICUs remain under-recognized compared to those in preterm infants. This study provides a comprehensive overview of their clinical features and underlying etiologies, highlighting a substantial proportion with identifiable causes, including genetic and non-genetic factors (e.g., neuromuscular and gastrointestinal). By outlining the diagnostic yield of key modalities and their clinical relevance, our findings offer practical guidance for early evaluation of feeding difficulties in this vulnerable population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Kovacic, K. et al. Pediatric feeding disorder: a nationwide prevalence study. J. Pediatr. 228, 126–131.e123 (2021).
Lee, K. W. et al. Evaluating the clinical symptoms of neonates with suspected dysphagia. J. Korean Acad. Rehabil. Med. 35, 265 (2011).
Hawdon, J., Beauregard, N., Slattery, J. & Kennedy, G. Identification of neonates at risk of developing feeding problems in infancy. Dev. Med. Child Neurol. 42, 235–239 (2000).
Edney, S. K., Jones, S. & Boaden, E. Screening for feeding difficulties in the neonatal unit: sensitivity and specificity of gestational age vs. medical history. J. Neonatal Nurs. 25, 116–120 (2019).
Macias, R., Peterson, D., Korkis, L., Edson, R. & Gall, R. Prevalence and impact of feeding-related events on hospital stay in preterm and term newborns. Adv. Neonatal Care 23, 541–546 (2023).
Delaney, A. L. & Arvedson, J. C. Development of swallowing and feeding: prenatal through first year of life. Dev. Disabil. Res. Rev. 14, 105–117 (2008).
Rybak, A. Organic and nonorganic feeding disorders. Ann. Nutr. Metab. 66, 16–22 (2015).
Kerzner, B. et al. A practical approach to classifying and managing feeding difficulties. Pediatrics 135, 344–353 (2015).
Gulati, I. K., Sultana, Z. & Jadcherla, S. R. Approach to feeding difficulties in neonates and infants: a comprehensive overview. Clin. Perinatol. 47, 265–276 (2020).
Park, J., Knafl, G., Thoyre, S. & Brandon, D. Factors associated with feeding progression in extremely preterm infants. Nurs. Res. 64, 159–167 (2015).
Jadcherla, S. R., Wang, M., Vijayapal, A. S. & Leuthner, S. R. Impact of prematurity and co-morbidities on feeding milestones in neonates: a retrospective study. J. Perinatol. 30, 201–208 (2010).
Palmer, M. M., Crawley, K. & Blanco, I. A. Neonatal oral-motor assessment scale: a reliability study. J. Perinatol. 13, 28–35 (1993).
Xiao, F. et al. Protocol of the China neonatal genomes project: an observational study about genetic testing on 100,000 neonates. Pediatr. Med. 4 (2021).
Dong, X. et al. Clinical exome sequencing as the first-tier test for diagnosing developmental disorders covering both CNV and SNV: a Chinese cohort. J. Med. Genet. 57, 558–566 (2020).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Yang, L. et al. Clinical features and underlying genetic causes in neonatal encephalopathy: a large cohort study. Clin. Genet. 98, 365–373 (2020).
Wang, H. et al. Genetic architecture in neonatal intensive care unit patients with congenital heart defects: a retrospective study from the China Neonatal Genomes Project. J. Med. Genet. 60, 247–253 (2023).
Huang, Z. et al. Comparison of genetic profiles of neonates in intensive care units conceived with or without assisted reproductive technology. JAMA Netw. Open 6, e236537 (2023).
Yang, L. et al. Perinatal features of Prader-Willi syndrome: a Chinese cohort of 134 patients. Orphanet J. Rare Dis. 15, 24 (2020).
Poskanzer, S. A., Hobensack, V. L., Ciciora, S. L. & Santoro, S. L. Feeding difficulty and gastrostomy tube placement in infants with down syndrome. Eur. J. Pediatr. 179, 909–917 (2020).
Kang, L. et al. A study on a cohort of 301 Chinese patients with isolated methylmalonic acidemia. J. Inherit. Metab. Dis. 43, 409–423 (2020).
Liang, L. et al. Evaluation of the clinical, biochemical, genotype and prognosis of mut-type methylmalonic acidemia in 365 Chinese cases. J. Med. Genet. 61, 8–17 (2023).
Chen, X. et al. Feeding difficulty is the dominant feature in 12 Chinese newborns with Chd7 pathogenic variants. BMC Med. Genet. 20, 93 (2019).
Johannesen, K. M. et al. Pura-related developmental and epileptic encephalopathy: phenotypic and genotypic spectrum. Neurol. Genet. 7, e613 (2021).
Maron, J. L. et al. Rapid whole-genomic sequencing and a targeted neonatal gene panel in infants with a suspected genetic disorder. Jama 330, 161–169 (2023).
Waldrop, M. A. et al. Gene therapy for spinal muscular atrophy: safety and early outcomes. Pediatrics 146, e20200729 (2020).
Mercuri, E. et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (Str1ve-Eu): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 20, 832–841 (2021).
Finkel, R. S. et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: final report of a phase 2, open-label, multicentre, dose-escalation study. Lancet Child Adolesc. Health 5, 491–500 (2021).
Finkel, R. S. et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 377, 1723–1732 (2017).
Baranello, G. et al. Risdiplam in type 1 spinal muscular atrophy. N. Engl. J. Med. 384, 915–923 (2021).
Lawlor, M. W. et al. Effects of gene replacement therapy with Resamirigene Bilparvovec (At132) on skeletal muscle pathology in X-linked myotubular myopathy: results from a substudy of the aspiro open-label clinical trial. EBioMedicine 99, 104894 (2024).
Shieh, P. B. et al. Safety and efficacy of gene replacement therapy for X-linked myotubular myopathy (aspiro): a multinational, open-label, dose-escalation trial. Lancet Neurol. 22, 1125–1139 (2023).
Talisman, S. et al. Neonatal intensive care admission for term neonates and subsequent childhood mortality: a retrospective linkage study. BMC Med. 21, 44 (2023).
Acknowledgements
We thank all patients, Yihao Zhou, and Shiyu Ji for their kind input. All phases of this study were supported by Sichuan Natural Science Foundation (2023NSFSC1604) and Future Medical Scientist Clinical Postdoctoral Program of Children’s Hospital of Fudan University.
Author information
Authors and Affiliations
Contributions
Tiantian Xiao and Wenhao Zhou conceptualized and designed the study, drafted the initial manuscript, and critically reviewed and revised the manuscript. Jing Wang designed the data collection instruments, collected data, carried out the initial analyses, drafted the initial manuscript, and critically reviewed and revised the manuscript. Jiaqi Mao, Bi Ze, Mengmeng Ge designed the data collection instruments, collected data, carried out the initial analyses, and critically reviewed and revised the manuscript. Liyuan Hu, Dong Xinran, Yulan Lu, and Bingbing Wu coordinated and supervised data collection, and critically reviewed and revised the manuscript for important intellectual content. Guoqiang Cheng and Lin Yang conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
This study protocol was reviewed and approved by Children’s Hospital of Fudan University, approval number 2015-169. Participation in the survey was voluntary, and participants consented by choosing “Yes—I agree to participate” and signing.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Mao, J., Ze, B. et al. Characterization and diagnosis of feeding difficulties in full-term neonates in NICUs: a multicenter study. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04257-w
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04257-w
This article is cited by
-
Analysis of factors affecting postoperative feeding intolerance in neonates following intestinal surgery
BMC Pediatrics (2026)
-
Pediatrics advances in 2024: choices in allergy, cardiology, critical care, endocrinology, gastroenterology, immunology, infectious diseases, neonatology, nephrology, neurology, nutrition, palliative care respiratory tract illnesses, and social media
Italian Journal of Pediatrics (2025)