Abstract
Background
The study aims to evaluate the effectiveness of low molecular weight heparin (LMWH) in reducing neonatal morbidity in pregnancies with early-onset fetal growth restriction (FGR).
Methods
A phase III, multicenter, triple-blind, parallel-arm randomized clinical trial conducted in two university hospitals in Spain. Singleton pregnancies with early-onset placental FGR (20 + 0–31 + 6 weeks at diagnosis) were randomized to receive bemiparin (3500 IU/0.2 mL/day) or placebo from diagnosis to delivery. The primary neonatal outcome was morbidity up to hospital discharge, assessed using the Morbidity Assessment Index for Newborns (MAIN) score. Analyses followed an intention-to-treat approach.
Results
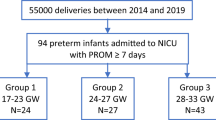
Fifty patients were randomized (25 per arm), with 49 included in the final analysis (23 LMWH, 26 placebo). Median gestational ages at inclusion were 28.7 weeks (LMWH) and 28.4 weeks (placebo). No significant differences were observed in MAIN scores between groups (171.5 vs. 290; p = 0.887; adjusted median difference 139.1 [95% CI −88.4 to 319.8]). NICU stay lengths were also similar (9 vs. 6.5 days; p = 0.738; adjusted median difference 1.08 [95% CI −0.74 to 13.4]).
Conclusion
Prophylactic LMWH started at diagnosis of early-onset FGR does not decrease neonatal morbidity or NICU stay duration.
Impact
-
Prophylactic LMWH started at the diagnosis of early-onset FGR does not decrease neonatal morbidity or NICU stay duration.
-
Its design is a randomized, triple-blind clinical trial, which provides high-quality evidence.
-
LMWH is often prescribed during pregnancy for various indications, and in the absence of effective alternatives, it is increasingly used empirically in such cases. Our findings do not support its use for reducing perinatal morbidity or NICU admission duration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures and appendices), will be shared with researchers who provide a methodologically sound proposal. Data will be shared, until a maximum of 15 years after study closure, to achieve aims in the approved proposal. Proposals should be directed to ffiguera@clinic.cat. To gain access, data requestors will need to sign a data access agreement.
References
Figueras, F. & Gratacós, E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn. Ther. 36, 86–98 (2014).
Baschat, A. A. et al. Predictors of neonatal outcome in early-onset placental dysfunction. Obstet. Gynecol. 109, 253–261 (2007).
Rosenberg, A. The IUGR newborn. Semin Perinatol. 32, 219–24 (2008).
Kesavan, K. & Devaskar, S. U. Intrauterine growth restriction: postnatal monitoring and outcomes. Pediatr. Clin. North Am. 66, 403–423 (2019).
Hossain, N. & Paidas, M. J. Adverse pregnancy outcome, the uteroplacental interface, and preventive strategies. Semin Perinatol. 31, 208–212 (2007).
Cole, T. J., Hey, E. & Richmond, S. The PREM score: a graphical tool for predicting survival in very preterm births. Arch. Dis. Child Fetal Neonatal Ed. 95, F14–F19 (2010).
Shomer, E. et al. Microvesicles of pregnant women receiving low molecular weight heparin improve trophoblast function. Thromb. Res. 137, 141–147 (2016).
Shomer, E. et al. Microvesicles of women with gestational hypertension and preeclampsia affect human trophoblast fate and endothelial function. Hypertension 62, 893–898 (2013).
González, A. et al. Treatment of early-onset fetal growth restriction with low molecular weight heparin does not prolong gestation: a randomized clinical trial. Am. J. Obstet. Gynecol. 232, 552.e1–552.e10 (2025).
Gordijn, S. J. et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 48, 333–339 (2016).
Arduini, D. & Rizzo, G. Normal values of Pulsatility Index from fetal vessels: a cross-sectional study on 1556 healthy fetuses. J. Perinat. Med. 18, 165–172 (1990).
Hadlock, F. P., Harrist, R. B., Sharman, R. S., Deter, R. L. & Park, S. K. Estimation of fetal weight with the use of head, body, and femur measurements-a prospective study. Am. J. Obstet. Gynecol. 151, 333–337 (1985).
Figueras, F. et al. Customized birthweight standards for a Spanish population. Eur. J. Obstet. Gynecol. Reprod. Biol. 136, 20–24 (2008).
Gómez, O. et al. Reference ranges for uterine artery mean pulsatility index at 11-41 weeks of gestation. Ultrasound Obstet. Gynecol. 32, 128–132 (2008).
Rodger M. A., Phillips, P., Kahn, S. R., James, A. H. & Konkle, B. A. PROSPER Investigators. Low-molecular-weight heparin to prevent postpartum venous thromboembolism. A pilot randomised placebo-controlled trial. Thromb Haemost. 113, 212–216 (2015).
Mazarico, E. et al. Study protocol for a randomised controlled trial: treatment of early intrauterine growth restriction with low molecular weight heparin (TRACIP). BMJ Open 8, e020501 (2018).
Robinson, H. P. & Fleming, J. E. E. A critical evaluation of sonar “crown-rump length” measurements. Br. J. Obstet. Gynaecol. 82, 702–710 (1975).
Bhide, A. et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet. Gynecol. 41, 233–239 (2013).
Crecimiento intrauterino restringido (online). Available at: https://fetalmedicinebarcelona.org/wp-content/uploads/2024/02/Defectos-del-crecimiento-fetal.pdf (2014).
MacOnes, G. A., Hankins, G. D. V., Spong, C. Y., Hauth, J. & Moore, T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet. Gynecol. 112, 661–666 (2008).
Tranquilli, A. L. et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens.4, 97–104 (2014).
Verma, A., Weir, A., Drummond, J. & Mitchell, B. F. Performance profile of an outcome measure: morbidity assessment index for newborns. J. Epidemiol. Community Health 59, 420 (2005).
Verma, A., Okun, N. B., Maguire, T. O. & Mitchell, B. F. Morbidity assessment index for newborns: a composite tool for measuring newborn health. Am. J. Obstet. Gynecol.181, 701–708 (1999).
CPMP/ICH/363/96. ICH E9 Statistical Principles for Clinical Trials. http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500002928 (2014).
Dodd, J. M., Mcleod, A., Windrim, R. C. & Kingdom, J. Antithrombotic therapy for improving maternal or infant health outcomes in women considered at risk of placental dysfunction. Cochrane Database Syst. Rev. 2013, CD006780 (2013).
Roberge, S. et al. Prevention of pre-eclampsia by low-molecular-weight heparin in addition to aspirin: a meta-analysis. Ultrasound Obstet. Gynecol. 47, 548–553 (2016).
Rodger, M. A. et al. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: a meta-analysis of individual patient data from randomised controlled trials. Lancet 388, 2629–2641 (2016).
Hansen, A. T. et al. Tinzaparin for the treatment of foetal growth retardation: an open-labelled randomized clinical trial. Thromb. Res. 170, 38–44 (2018).
Shirazi, M. et al. Therapeutic role of enoxaparin in intra-uterine growth restriction: a randomized clinical trial. J. Gynecol. Obstet. Hum. Reprod. 50, 102070 (2021).
Yu, Y. H., Shen, L. Y., Zou, H., Wang, Z. J. & Gong, S. P. Heparin for patients with growth restricted fetus: a prospective randomized controlled trial. J. Matern. Fetal Neonatal Med. 23, 980–987 (2010).
Kingdom, J. C. P. et al. Unfractionated heparin for second trimester placental insufficiency: a pilot randomized trial. J. Thromb. Haemost. 9, 1483–1492 (2011).
Lees, C. C. et al. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 56, 298–312 (2020).
Azizi, M. et al. Comparison of biological activities of two low molecular weight heparins in 10 healthy volunteers. Br. J. Clin. Pharm. 40, 577–584 (1995).
Sánchez-Ferrer, C. F. Bemiparin: pharmacological profile. Drugs 70, 19–23 (2010).
Groom, K. M. & David, A. L. The role of aspirin, heparin, and other interventions in the prevention and treatment of fetal growth restriction. Am. J. Obstet. Gynecol. 218, S829–S840 (2018).
Nawathe, A. & David, A. L. Prophylaxis and treatment of foetal growth restriction. Best. Pr. Res. Clin. Obstet. Gynaecol. 49, 66–78 (2018).
Funding
This work has been funded by the Plan Estatal de I+D+i (Government R&D&I Plan) and Instituto de Salud Carlos III- Subdirección General de Evaluación y Fomento de la Investigación Sanitaria (Carlos III Health Institute – General Subdirectorate for Assessment and Promotion of Research), projects PI16/00151 and PI16/595, and the European Regional Development Fund (FEDER).
Author information
Authors and Affiliations
Contributions
Alba González, Anna Peguero, Eva Meler, Narcís Masoller, Daniel Oros, Judit Hidalgo and Patricia Ibáñez-Burillo were coordinators at their respective sites and collected the data. Marta Camprubí, Carlota Rovira, Maria Dolores Gómez Roig, Jon Schoorlemmer and Maria Dolors Tàssies and contributed data or analysis tools. Edurne Mazarico is the general coordinator and principal investigator of the project, conceived and designed the analysis and wrote the paper. Francesc Figueras was consultant and co-principal investigator of the study, conceived and designed the analysis and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
All participants provided individual informed consent. The trial was approved by the Ethics Committee of each participant centre (AC-09-17).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
González, A., Peguero, A., Meler, E. et al. Neonatal morbidity in early-onset fetal growth restriction with and without anticoagulant therapy. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04347-9
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04347-9