Abstract
Background
Exogenous corticosteroid exposure is common in premature infants and can interfere with normal developmental processes. It remains unknown if there are long-term alterations to cardiometabolic health following antenatal corticosteroid (ANCS) plus postnatal corticosteroid (PNCS) exposure.
Methods
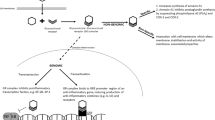
Pregnant mice received intraperitoneal (IP) injections of dexamethasone 0.5 mg/kg on gestational days 15–16. Pups delivered naturally. On postnatal days (PD) 1–3, offspring received IP injections of dexamethasone 1.2 mg/kg or saline control. On PD 90, offspring were euthanized and organs harvested for study.
Results
Compared to ANCS alone, offspring exposed to ANCS + PNCS had decreased body weights, and lungs had alveolar simplification with increased mean linear intercept and decreased radial alveolar count. Exposure to ANCS + PNCS increased cardiomyocyte diameter compared to ANCS alone. Mice exposed to ANCS + PNCS had attenuation in liver mRNA levels in genes responsible for energy homeostasis including adiponectin, peroxisome proliferator-activated receptor gamma coactivator 1-alpha, and sirtuin 1, and alterations in free fatty acids.
Conclusions
Young adult mice exposed to ANCS + PNCS compared to ANCS alone have evidence of lung simplification, cardiomyocyte hypertrophy, and metabolism-related gene alterations in liver. This study is limited by the lack of a control group with no exposure to corticosteroids.
Impact
-
Antenatal + short course of postnatal corticosteroid exposure (3 days) results in long-term multi-organ system changes in adult mice.
-
Mice exposed to antenatal + postnatal corticosteroids exhibit impaired alveolarization, cardiomyocyte hypertrophy, and metabolic alterations in young adulthood.
-
Corticosteroids are a common exposure in premature infants and may contribute to long-term morbidities in this population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data are available by written request to jdillard@akronchildrens.org.
References
Crump, C. An overview of adult health outcomes after preterm birth. Early Hum. Dev. 150, 105187, https://doi.org/10.1016/j.earlhumdev.2020.105187 (2020).
Lurbe, E. & Ingelfinger, J. Developmental and early life origins of cardiometabolic risk factors: novel findings and implications. Hypertension 77, 308–318, https://doi.org/10.1161/HYPERTENSIONAHA.120.14592 (2021).
Rog-Zielinska, E. A., Richardson, R. V., Denvir, M. A. & Chapman, K. E. Glucocorticoids and foetal heart maturation; implications for prematurity and foetal programming. J. Mol. Endocrinol. 52, R125–R135, https://doi.org/10.1530/JME-13-0204 (2014).
Asztalos, E. Antenatal corticosteroids: a risk factor for the development of chronic disease. J. Nutr. Metab. 2012, 930591, https://doi.org/10.1155/2012/930591 (2012).
Busada, J. T. & Cidlowski, J. A. Mechanisms of glucocorticoid action during development. Curr. Top. Dev. Biol. 125, 147–170, https://doi.org/10.1016/bs.ctdb.2016.12.004 (2017).
Braun, T., Challis, J. R., Newnham, J. P. & Sloboda, D. M. Early-life glucocorticoid exposure: the hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk. Endocr. Rev. 34, 885–916, https://doi.org/10.1210/er.2013-1012 (2013).
Committee on Obstetric, P Committee opinion No. 713: antenatal corticosteroid therapy for fetal maturation. Obstet. Gynecol. 130, e102–e109, https://doi.org/10.1097/AOG.0000000000002237 (2017).
Kemp, M. W. et al. Efficacy and safety of antenatal steroids. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R825–R839, https://doi.org/10.1152/ajpregu.00193.2017 (2018).
Roberts, D., Brown, J., Medley, N. & Dalziel, S. R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 3, CD004454, https://doi.org/10.1002/14651858.CD004454.pub3 (2017).
Doyle, L. W., Cheong, J. L., Hay, S., Manley, B. J. & Halliday, H. L. Late (>/= 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 11, CD001145, https://doi.org/10.1002/14651858.CD001145.pub5 (2021).
Lok, I. M. et al. Effects of postnatal corticosteroids on lung development in newborn animals. A systematic review. Pediatr. Res. https://doi.org/10.1038/s41390-024-03114-6 (2024).
Harris, C. et al. Postnatal dexamethasone exposure and lung function in adolescents born very prematurely. PLoS One 15, e0237080, https://doi.org/10.1371/journal.pone.0237080 (2020).
Vrselja, A., Pillow, J. J. & Black, M. J. Effect of preterm birth on cardiac and cardiomyocyte growth and the consequences of antenatal and postnatal glucocorticoid treatment. J. Clin. Med. 10 https://doi.org/10.3390/jcm10173896 (2021).
Festing, M. F. & Altman, D. G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 43, 244–258, https://doi.org/10.1093/ilar.43.4.244 (2002).
Li, H., Yuan, X., Tang, J. & Zhang, Y. Lipopolysaccharide disrupts the directional persistence of alveolar myofibroblast migration through EGF receptor. Am. J. Physiol. Lung Cell Mol. Physiol. 302, L569–L579, https://doi.org/10.1152/ajplung.00217.2011 (2012).
Cooney, T. P. & Thurlbeck, W. M. The radial alveolar count method of Emery and Mithal: a reappraisal 1–postnatal lung growth. Thorax 37, 572–579, https://doi.org/10.1136/thx.37.8.572 (1982).
Emery, J. L. & Mithal, A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch. Dis. Child 35, 544–547, https://doi.org/10.1136/adc.35.184.544 (1960).
Xu, J. et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ. Res. 98, 342–350, https://doi.org/10.1161/01.RES.0000202804.84885.d0 (2006).
Wallner, M. et al. Acute catecholamine exposure causes reversible myocyte injury without cardiac regeneration. Circ. Res 119, 865–879, https://doi.org/10.1161/CIRCRESAHA.116.308687 (2016).
Hillman, N. H. et al. Dose of budesonide with surfactant affects lung and systemic inflammation after normal and injurious ventilation in preterm lambs. Pediatr. Res. 88, 726–732, https://doi.org/10.1038/s41390-020-0809-6 (2020).
Cummings, J. J., Pramanik, A. K., Committee On, F. & Newborn. Postnatal corticosteroids to prevent or treat chronic lung disease following preterm birth. Pediatrics 149 https://doi.org/10.1542/peds.2022-057530 (2022).
Jobe, A. H., Milad, M. A., Peppard, T. & Jusko, W. J. Pharmacokinetics and pharmacodynamics of intramuscular and oral betamethasone and dexamethasone in reproductive age women in India. Clin. Transl. Sci. 13, 391–399, https://doi.org/10.1111/cts.12724 (2020).
Tijsseling, D. et al. Neonatal corticosteroid therapy affects growth patterns in early infancy. PLoS One 13, e0192162, https://doi.org/10.1371/journal.pone.0192162 (2018).
Regan, F. M., Cutfield, W. S., Jefferies, C., Robinson, E. & Hofman, P. L. The impact of early nutrition in premature infants on later childhood insulin sensitivity and growth. Pediatrics 118, 1943–1949, https://doi.org/10.1542/peds.2006-0733 (2006).
Singhal, A. Early nutrition and long-term cardiovascular health. Nutr. Rev. 64, S44–S49, https://doi.org/10.1301/nr.2006.may.s44-s49 (2006). discussion S72-91.
Singhal, A., Cole, T. J., Fewtrell, M., Deanfield, J. & Lucas, A. Is slower early growth beneficial for long-term cardiovascular health? Circulation 109, 1108–1113, https://doi.org/10.1161/01.CIR.0000118500.23649.DF (2004).
Qin, G. et al. Postnatal dexamethasone, respiratory and neurodevelopmental outcomes at two years in babies born extremely preterm. PLoS One 12, e0181176, https://doi.org/10.1371/journal.pone.0181176 (2017).
Harris, C. et al. Effect of dexamethasone exposure on the neonatal unit on the school age lung function of children born very prematurely. PLoS One 13, e0200243, https://doi.org/10.1371/journal.pone.0200243 (2018).
Evans, N. Cardiovascular effects of dexamethasone in the preterm infant. Arch. Dis. Child Fetal Neonatal Ed. 70, F25–F30, https://doi.org/10.1136/fn.70.1.f25 (1994).
Gill, A. W., Warner, G. & Bull, L. Iatrogenic neonatal hypertrophic cardiomyopathy. Pediatr. Cardiol. 17, 335–339, https://doi.org/10.1007/s002469900075 (1996).
Werner, J. C. et al. Hypertrophic cardiomyopathy associated with dexamethasone therapy for bronchopulmonary dysplasia. J. Pediatr. 120, 286–291, https://doi.org/10.1016/s0022-3476(05)80446-9 (1992).
Zecca, E. et al. Cardiac adverse effects of early dexamethasone treatment in preterm infants: a randomized clinical trial. J. Clin. Pharm. 41, 1075–1081, https://doi.org/10.1177/00912700122012670 (2001).
Skelton, R., Gill, A. B. & Parsons, J. M. Cardiac effects of short course dexamethasone in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 78, F133–F137, https://doi.org/10.1136/fn.78.2.f133 (1998).
Ohning, B. L., Fyfe, D. A. & Riedel, P. A. Reversible obstructive hypertrophic cardiomyopathy after dexamethasone therapy for bronchopulmonary dysplasia. Am. Heart J. 125, 253–256, https://doi.org/10.1016/0002-8703(93)90089-r (1993).
de Vries, W. B. et al. Alterations in adult rat heart after neonatal dexamethasone therapy. Pediatr. Res 52, 900–906, https://doi.org/10.1203/00006450-200212000-00015 (2002).
Bal, M. P. et al. Histopathological changes of the heart after neonatal dexamethasone treatment: studies in 4-, 8-, and 50-week-old rats. Pediatr. Res 66, 74–79, https://doi.org/10.1203/PDR.0b013e3181a283a0 (2009).
Niu, Y., Herrera, E. A., Evans, R. D. & Giussani, D. A. Antioxidant treatment improves neonatal survival and prevents impaired cardiac function at adulthood following neonatal glucocorticoid therapy. J. Physiol. 591, 5083–5093, https://doi.org/10.1113/jphysiol.2013.258210 (2013).
Bal, M. P. et al. Long-term cardiovascular effects of neonatal dexamethasone treatment: hemodynamic follow-up by left ventricular pressure-volume loops in rats. J. Appl Physiol. (1985) 104, 446–450, https://doi.org/10.1152/japplphysiol.00951.2007 (2008).
Kamphuis, P. J. et al. Reduced life expectancy in rats after neonatal dexamethasone treatment. Pediatr. Res 61, 72–76, https://doi.org/10.1203/01.pdr.0000249980.95264.dd (2007).
Jiang, X. et al. Effects of neonatal dexamethasone administration on cardiac recovery ability under ischemia-reperfusion in 24-wk-old rats. Pediatr. Res. 80, 128–135, https://doi.org/10.1038/pr.2016.54 (2016).
Cardoso, R. C. & Padmanabhan, V. Prenatal steroids and metabolic dysfunction: lessons from sheep. Annu. Rev. Anim. Biosci. 7, 337–360, https://doi.org/10.1146/annurev-animal-020518-115154 (2019).
Fowden, A. L., Giussani, D. A. & Forhead, A. J. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 21, 29–37, https://doi.org/10.1152/physiol.00050.2005 (2006).
Jellyman, J. K., Valenzuela, O. A. & Fowden, A. L. HORSE SPECIES SYMPOSIUM: glucocorticoid programming of hypothalamic-pituitary-adrenal axis and metabolic function: Animal studies from mouse to horse. J. Anim. Sci. 93, 3245–3260, https://doi.org/10.2527/jas.2014-8612 (2015).
Leone, T. C. et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 3, e101, https://doi.org/10.1371/journal.pbio.0030101 (2005).
Besse-Patin, A. et al. Estrogen signals through peroxisome proliferator-activated receptor-gamma coactivator 1alpha to reduce oxidative damage associated with diet-induced fatty liver disease. Gastroenterology 152, 243–256, https://doi.org/10.1053/j.gastro.2016.09.017 (2017).
Estall, J. L. et al. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression. Diabetes 58, 1499–1508, https://doi.org/10.2337/db08-1571 (2009).
Kleiner, S. et al. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc. Natl. Acad. Sci. USA 109, 9635–9640, https://doi.org/10.1073/pnas.1207287109 (2012).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217, https://doi.org/10.1016/j.cell.2013.05.039 (2013).
Zhang, Y. et al. Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: role of AMPK, Sirt1, and mitochondrial function. Free Radic. Biol. Med. 71, 208–220, https://doi.org/10.1016/j.freeradbiomed.2014.03.018 (2014).
Lemieux, H., Vazquez, E. J., Fujioka, H. & Hoppel, C. L. Decrease in mitochondrial function in rat cardiac permeabilized fibers correlates with the aging phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 65, 1157–1164, https://doi.org/10.1093/gerona/glq141 (2010).
Owesny, P. & Grune, T. The link between obesity and aging - insights into cardiac energy metabolism. Mech. Ageing Dev. 216, 111870. https://doi.org/10.1016/j.mad.2023.111870 (2023).
Balsan, G. A., Vieira, J. L., Oliveira, A. M. & Portal, V. L. Relationship between adiponectin, obesity and insulin resistance. Rev. Assoc. Med. Bras. (1992) 61, 72–80, https://doi.org/10.1590/1806-9282.61.01.072 (2015).
Jung, U. J. & Choi, M. S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 15, 6184–6223, https://doi.org/10.3390/ijms15046184 (2014).
Hulthe, J., Hulten, L. M. & Fagerberg, B. Low adipocyte-derived plasma protein adiponectin concentrations are associated with the metabolic syndrome and small dense low-density lipoprotein particles: atherosclerosis and insulin resistance study. Metabolism 52, 1612–1614, https://doi.org/10.1016/s0026-0495(03)00313-5 (2003).
Crume, T. L. et al. The long-term impact of intrauterine growth restriction in a diverse U.S. cohort of children: the EPOCH study. Obesity (Silver Spring) 22, 608–615, https://doi.org/10.1002/oby.20565 (2014).
Ordonez-Diaz, M. D. et al. Plasma adipokines profile in prepubertal children with a history of prematurity or extrauterine growth restriction. Nutrients 12 https://doi.org/10.3390/nu12041201 (2020).
Dai, Y. et al. Prenatal prednisone exposure impacts liver development and function in fetal mice and its characteristics. Toxicol. Sci. 199, 63–80, https://doi.org/10.1093/toxsci/kfae027 (2024).
Nair, A. B. & Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31, https://doi.org/10.4103/0976-0105.177703 (2016).
Schittny, J. C. Development of the lung. Cell Tissue Res. 367, 427–444, https://doi.org/10.1007/s00441-016-2545-0 (2017).
Nguyen, T. & Jordan, B. K. Let’s talk about dex: when do the benefits of dexamethasone for prevention of bronchopulmonary dysplasia outweigh the risks?. Newborn (Clarksville) 1, 91–96, https://doi.org/10.5005/jp-journals-11002-0009 (2022).
Dutta, S. & Sengupta, P. Men and mice: relating their ages. Life Sci. 152, 244–248, https://doi.org/10.1016/j.lfs.2015.10.025 (2016).
Effect of corticosteroids for fetal maturation on perinatal outcomes NIH consensus development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 273, 413–418, https://doi.org/10.1001/jama.1995.03520290065031 (1995).
Kim, Y. E., Park, W. S., Sung, D. K., Ahn, S. Y. & Chang, Y. S. Antenatal betamethasone enhanced the detrimental effects of postnatal dexamethasone on hyperoxic lung and brain injuries in newborn rats. PLoS One 14, e0221847, https://doi.org/10.1371/journal.pone.0221847 (2019).
Ersek, A. et al. Strain dependent differences in glucocorticoid-induced bone loss between C57BL/6J and CD-1 mice. Sci. Rep. 6, 36513. https://doi.org/10.1038/srep36513 (2016).
Hodes, G. E. et al. Strain differences in the effects of chronic corticosterone exposure in the hippocampus. Neuroscience 222, 269–280, https://doi.org/10.1016/j.neuroscience.2012.06.017 (2012).
Shelton, E. L. et al. Effects of antenatal betamethasone on preterm human and mouse ductus arteriosus: comparison with baboon data. Pediatr. Res. 84, 458–465, https://doi.org/10.1038/s41390-018-0006-z (2018).
Zhang, H. et al. The angiogenic factor midkine is regulated by dexamethasone and retinoic acid during alveolarization and in alveolar epithelial cells. Respir. Res. 10, 77, https://doi.org/10.1186/1465-9921-10-77 (2009).
Krishnan, A. et al. A detailed comparison of mouse and human cardiac development. Pediatr. Res. 76, 500–507, https://doi.org/10.1038/pr.2014.128 (2014).
Wessels, A. & Sedmera, D. Developmental anatomy of the heart: a tale of mice and man. Physiol. Genom. 15, 165–176, https://doi.org/10.1152/physiolgenomics.00033.2003 (2003).
Kaffe, E. et al. Humanized mouse liver reveals endothelial control of essential hepatic metabolic functions. Cell 186, 3793–3809 e3726, https://doi.org/10.1016/j.cell.2023.07.017 (2023).
Vickers, M. H. Developmental programming and transgenerational transmission of obesity. Ann. Nutr. Metab. 64, 26–34, https://doi.org/10.1159/000360506 (2014).
Buescher, J. L. et al. Evidence for transgenerational metabolic programming in Drosophila. Dis. Model Mech. 6, 1123–1132, https://doi.org/10.1242/dmm.011924 (2013).
Zhu, Z., Cao, F. & Li, X. Epigenetic programming and fetal metabolic programming. Front Endocrinol. (Lausanne) 10, 764, https://doi.org/10.3389/fendo.2019.00764 (2019).
Funding
This study was funded by grants from the Saint Louis University School of Medicine President’s Research Fund and Cardinal Glennon Children’s Foundation. The funders played no role in the design, analysis, and reporting of the study.
Author information
Authors and Affiliations
Contributions
Conception and design: Julie Dillard, Noah Hillman; Acquisition of data: Julie Dillard, Emily Royse; Analysis and interpretation of data: Julie Dillard, Emily Royse, Noah Hillman; Manuscript preparation: Julie Dillard, Emily Royse, Noah Hillman; All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dillard, J.A., Royse, E.X. & Hillman, N.H. Long-term multi-organ system abnormalities in mice exposed to antenatal and postnatal corticosteroids. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04373-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04373-7