Abstract
Background
This study aims to explore the causal relationship between gut microbiota and childhood obesity and to assess the potential mediating role of blood metabolites in this relationship.
Methods
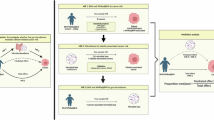
This study covering 473 gut microbiota and 1400 metabolites. The bidirectional two-sample Mendelian Randomization method is employed, with Inverse Variance Weighted as the main statistical approach, to assess the causal relationships between gut microbiota, metabolites and Childhood Obesity.
Results
The study found significant causal associations between 12 types of gut microbiota and childhood obesity, with 7 microbiota showing a negative correlation and 5 acting as risk factors for obesity. Bacillaceae A showed the strongest association with childhood obesity (OR = 0.0481, P = 0.0126), while Eubacterium Q was identified as a major risk bacterium for obesity (OR = 5.4330, P = 0.0452). Additionally, 18 metabolites were found to be associated with childhood obesity, with 6-bromotryptophan being the strongest negatively correlated metabolite (OR = 0.5680, P = 0.00023) and methylsuccinate being the strongest positively correlated risk metabolite (OR = 1.6987, P = 0.0383). Mediation analysis identified 10 significant pathways, highlighting Adrenate (22:4n6) as a mediator for the gut microbiota UBA2922 sp900313925 in promoting childhood obesity (mediation effect: 0.106, mediation proportion: 10.94%), and X-12830 as a mediator for K10 sp001941205 in inhibiting childhood obesity (mediation effect: −0.0772, mediation proportion: 13.68%).
Conclusion
This study provides novel evidence of the causal roles of gut microbiota and blood metabolites in childhood obesity. The identification of key metabolites mediating the effects of gut microbiota on obesity risk offers potential targets for future interventions and therapeutic strategies.
Impact
-
Analysis revealed 12 gut microbiota causally related to childhood obesity, with seven negatively correlated and five identified as risk factors. Bacillaceae A (protective) and Eubacterium Q (risk) were notably associated.
-
Among 1400 metabolites studied, 18 showed causal relationships with obesity, notably 6-bromotryptophan (protective) and methylsuccinate (risk factor).
-
Mediation analysis identified 10 significant causal pairings between gut microbiota and blood metabolites, with Adrenate (22:4n6) mediating the promotion of childhood obesity by UBA2922 sp900313925, and X-12830 mediating the inhibition of childhood obesity by K10 sp001941205.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available in the Finngen database (https://www.finngen.fi/en) and IEU OpenGWAS (https://gwas.mrcieu.ac.uk/).
References
Pulungan, A. B. et al. Childhood obesity as a global problem: a cross-sectional survey on global awareness and national program implementation. J. Clin. Res. Pediatr. Endocrinol. 16, 31–40 (2024).
Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390, 2627–2642 (2017).
Zhang, X. et al. Global prevalence of overweight and obesity in children and adolescents: a systematic review and meta-analysis. Jama Pediatr. 178, 800–813 (2024).
Zeljkovic, A., Vekic, J. & Stefanovic, A. Obesity and dyslipidemia in early life: impact on cardiometabolic risk. Metabolism 156, 155919 (2024).
Salama, M., Balagopal, B., Fennoy, I. & Kumar, S. Childhood obesity, diabetes. and cardiovascular disease risk. J. Clin. Endocrinol. Metab. 108, 3051–3066 (2023).
Chen, Y. et al. Obesity and risk of depressive disorder in children and adolescents: a meta-analysis of observational studies. Child. Care. Health Dev. 50, e13237 (2024).
Pan, Y. & Jiao, F. Y. Link between childhood obesity and gut microbiota. World J. Gastroenterol. 30, 3560–3563 (2024).
Nathan, N. N., Philpott, D. J. & Girardin, S. E. The intestinal microbiota: from health to disease, and back. Microbes Infect. 23, 104849 (2021).
Islam, M. M., Islam, M. M., Rahman, M. A., Ripon, M. & Hossain, M. S. Gut microbiota in obesity and related complications: unveiling the complex interplay. Life Sci. 334, 122211 (2023).
Koliada, A. et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol 17, 120 (2017).
Puljiz, Z. et al. Obesity, gut microbiota, and metabolome: from pathophysiology to nutritional interventions. Nutrients 15, 2236 (2023).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA 326, 1614–1621 (2021).
Qin, Y. et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat. Genet. 54, 134–142 (2022).
Chen, Y. et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat. Genet. 55, 44–53 (2023).
Bradfield, J. P. et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat. Genet. 44, 526–531 (2012).
Ganapathiraju, M. K., Subramanian, S., Chaparala, S. & Karunakaran, K. B. A reference catalog of DNA palindromes in the human genome and their variations in 1000 genomes. Hum. Genome Var. 7, 40 (2020).
Burgess, S., Small, D. S. & Thompson, S. G. A review of instrumental variable estimators for mendelian randomization. Stat. Methods Med. Res. 26, 2333–2355 (2017).
Wang, J., Zhu, N., Su, X., Gao, Y. & Yang, R. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells 12, 793 (2023).
Han, H. et al. From gut microbiota to host appetite: gut microbiota-derived metabolites as key regulators. Microbiome 9, 162 (2021).
Blaak, E. E. et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 11, 411–455 (2020).
Longo, S., Rizza, S. & Federici, M. Microbiota-gut-brain axis: relationships among the vagus nerve, gut microbiota, obesity, and diabetes. Acta Diabetol. 60, 1007–1017 (2023).
Wang, Y. et al. The gut microbiota reprograms intestinal lipid metabolism through long noncoding RNA Snhg9. Science 381, 851–857 (2023).
Tokuhara, D. et al. A comprehensive understanding of the gut mucosal immune system in allergic inflammation. Allergol. Int. 68, 17–25 (2019).
Virtue, A. T. et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci. Transl. Med. 11, eaav1892 (2019).
Scheithauer, T. et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 11, 571731 (2020).
Zheng, R. et al. The metabolomic profiling of total fat and fat distribution in a multi-cohort study of women and men. Sci. Rep. 13, 11129 (2023).
Kubota, Y. et al. Old-age-induced obesity reversed by a methionine-deficient diet or oral administration of recombinant methioninase-producing Escherichia coli in C57Bl/6 mice. Aging 15, 4642–4648 (2023).
Su, X., Gao, Y. & Yang, R. Gut microbiota-derived tryptophan metabolites maintain gut and systemic homeostasis. Cells 11, 2296 (2022).
Liu, R. et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 23, 7 (2017).
Acknowledgements
We would like to thank all the authors who contributed to the drafting of the manuscript. Funding support: This study was funded by the Guangxi Science and Technology Program Project (Guike AD22035121).
Author information
Authors and Affiliations
Contributions
Ji-Gan Wang designed the study and interpreted the results. Ji-Gan Wang and Xiu-Hua Pan were responsible for the conceptualization, methodology, data analysis, and manuscript writing. Xiu-Hua Pan and Yan Li participated in supervising the study, project management, and funding acquisition, and reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
In this MR study, we used publicly available aggregate data; therefore, no separate ethical approval is required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, JG., Pan, XH. & Li, Y. Causal relationship between gut microbiota and blood metabolites with childhood Obesity: a Mendelian randomization study. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04414-1
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04414-1