Abstract
Background
Traditionally, platelets were thought to originate solely in the bone marrow, but emerging evidence now indicates that the lung is a major site of platelet generation. Our previous work, along with studies by others, has revealed decreased platelet counts and enhanced platelet activation in BPD patients, linking platelets to the disease’s pathogenesis.
Methods
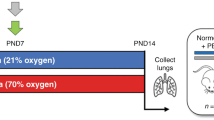
We investigated the protective effects and underlying mechanisms of umbilical cord blood (UCB) platelets in mitigating hyperoxia-induced lung injury in neonatal rats and in vitro.
Results
Compared with platelet-poor plasma (PPP), UCB-derived platelets (PLT) treatment preserved lung development, as evidenced by an increased density of secondary crests and reduced small arterial wall thickness. Given that secondary crest formation depends on myofibroblast activity, we further examined the cellular function of myofibroblasts isolated from the lungs of control and hyperoxic mice. We found that hyperoxia impaired myofibroblast function, particularly their migration capacity, which can be prevented by UCB platelet lysate.
Conclusion
These findings suggest that UCB PLT preserve lung development by protecting myofibroblast migratory capacity, offering new insights into BPD pathogenesis and potential therapeutic strategies.
Impact
-
Hyperoxia impairs myofibroblast function, particularly their migratory capacity, which can be prevented by umbilical cord blood platelet lysate.
-
Umbilical cord blood platelets preserve lung development in hyperoxia-exposed newborn rats by increasing the density of secondary crests and mitigating vascular remodeling in small arteries.
-
These findings underscore the promising therapeutic potential of umbilical cord blood-derived platelets for the management of BPD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Gilfillan, M., Bhandari, A. & Bhandari, V. Diagnosis and management of bronchopulmonary dysplasia. BMJ 375, n1974 (2021).
Abiramalatha, T. et al. Interventions to prevent bronchopulmonary dysplasia in preterm neonates: an umbrella review of systematic reviews and meta-analyses. JAMA Pediatr. 176, 502–516 (2022).
Durlak, W. & Thébaud, B. Bpd: latest strategies of prevention and treatment. Neonatology 121, 596–607 (2024).
Voynow, J. A. “New” bronchopulmonary dysplasia and chronic lung disease. Paediatr. Respir. Rev. 24, 17–18 (2017).
Wu, T. J. et al. Role of myeloperoxidase, oxidative stress, and inflammation in bronchopulmonary dysplasia. Antioxidants 13, 889 (2024).
McEvoy, C. T. & Durand, M. Anti-vascular endothelial growth factor antagonists: a potential primary prevention for bronchopulmonary dysplasia? Am. J. Respir. Crit. Care Med. 197, 703–704 (2018).
Yumani, D. F. J., Walschot, F. H., Lafeber, H. N. & van Weissenbruch, M. M. Associations between bronchopulmonary dysplasia, insulin-like growth factor I and nutrition. Nutrients 16, 957 (2024).
Gilfillan, M. & Bhandari, V. Moving bronchopulmonary dysplasia research from the bedside to the bench. Am. J. Physiol. Lung Cell. Mol. Physiol. 322, L804–l821 (2022).
Lefrançais, E. et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544, 105–109 (2017).
Chen, X. et al. Close association between platelet biogenesis and alveolarization of the developing lung. Front. Pediatr. 9, 625031 (2021).
Huang, Z. et al. Platelets are indispensable for alveolar development in neonatal mice. Front. Pediatr. 10, 943054 (2022).
Cortesi, V. et al. Why might cord blood be a better source of platelets for transfusion to neonates? Blood Transfus. 22, 292–302 (2024).
Chen, X. et al. Vascular and pulmonary effects of ibuprofen on neonatal lung development. Respir. Res. 24, 39 (2023).
Chen, X. et al. Inhibition of lysophosphatidic acid receptor 2 attenuates neonatal chronic lung disease in mice by preserving vascular and alveolar development. Eur. J. Pharmacol. 985, 177120 (2024).
Chen, X. et al. Bone morphogenetic protein 9 protects against neonatal hyperoxia-induced impairment of alveolarization and pulmonary inflammation. Front. Physiol. 8, 486 (2017).
Rodríguez-Castillo, J. A. et al. Understanding alveolarization to induce lung regeneration. Respir. Res. 19, 148 (2018).
Li, T. et al. Right ventricular function indices and platelet parameters for early prediction value of bronchopulmonary dysplasia: a retrospective study. BMC Pediatr. 24, 391 (2024).
Wang, X., Ma, Y., Wang, S., Dong, W. & Lei, X. Platelet is the early predictor of bronchopulmonary dysplasia in very premature infants: an observational cohort study. BMC Pulm. Med. 22, 109 (2022).
Golebiewska, E. M. & Poole, A. W. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 29, 153–162 (2015).
Tsukiji, N. et al. Platelets play an essential role in murine lung development through Clec-2/podoplanin interaction. Blood 132, 1167–1179 (2018).
Rafii, S. et al. Platelet-derived Sdf-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration. Nat. Cell Biol. 17, 123–136 (2015).
Johnson, J. et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J. Extracell. Vesicles 12, e12332 (2023).
Wu, S. et al. Extracellular vesicles meet mitochondria: potential roles in regenerative medicine. Pharmacol. Res. 206, 107307 (2024).
Kawada, K. et al. Cell migration is regulated by platelet-derived growth factor receptor endocytosis. Mol. Cell. Biol. 29, 4508–4518 (2009).
Yamaguchi, N. & Knaut, H. Focal adhesion-mediated cell anchoring and migration: from in vitro to in vivo. Development 149, dev200647 (2022).
Chastney, M. R., Kaivola, J., Leppänen, V. M. & Ivaska, J. The role and regulation of integrins in cell migration and invasion. Nat. Rev. Mol. Cell Biol. 26, 147–167 (2025).
Cheng, N. C., Tu, Y. K., Lee, N. H. & Young, T. H. Influence of human platelet lysate on extracellular matrix deposition and cellular characteristics in adipose-derived stem cell sheets. Front. Cell Dev. Biol. 8, 558354 (2020).
Scopelliti, F. et al. Platelet lysate promotes the expansion of t regulatory cells that favours in vitro wound healing by increasing keratinocyte migration and fibroblast production of extracellular matrix components. Eur. J. Dermatol. 30, 3–11 (2020).
Wu, C. Y. et al. The role of phosphoinositide-regulated actin reorganization in chemotaxis and cell migration. Br. J. Pharmacol. 171, 5541–5554 (2014).
Scopelliti, F. et al. Platelet lysate converts M (Ifnγ+Lps) macrophages in Cd206(+) Tgf-Β(+) Arginase(+) M2-Like macrophages that affect fibroblast activity and T lymphocyte migration. J. Tissue Eng. Regen. Med. 15, 788–797 (2021).
Amelio, G. S. et al. Endothelial dysfunction in preterm infants: the hidden legacy of uteroplacental pathologies. Front. Pediatr. 10, 1041919 (2022).
Herzog, B. H. et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet Clec-2. Nature 502, 105–109 (2013).
Song, L., Li, K., Chen, H. & Xie, L. Cell cross-talk in alveolar microenvironment: from lung injury to fibrosis. Am. J. Respir. Cell Mol. Biol. 71, 30–42 (2024).
Curley, A. et al. Randomized trial of platelet-transfusion thresholds in neonates. N. Engl. J. Med. 380, 242–251 (2019).
Sola-Visner, M. C. Platelet transfusions in neonates - less is more. N. Engl. J. Med. 380, 287–288 (2019).
Davenport, P. & Sola-Visner, M. Platelets in the neonate: not just a small adult. Res. Pract. Thromb. Haemost. 6, e12719 (2022).
Davenport, P. E. et al. Developmental differences between neonatal and adult platelets in regard to immune protein content and regulation of monocyte inflammatory responses. Blood 142, 699 (2023).
Acknowledgements
The authors gratefully acknowledge Dr. Lin Yi for her assistance in acquiring the umbilical cord blood.
Funding
This study is supported by National Natural Science Foundation of China (82101803 to X.C. and 82371707 to C.Y.), Guangdong Basic and Applied Basic Research Foundation (2020B1515120034 to C.Y.), Sanming Project of Medicine in Shenzhen (SZSM202211001), and Shenzhen Key Laboratory of Maternal and Child Health and Diseases (ZDSYS20230626091559006).
Author information
Authors and Affiliations
Contributions
C.Y., Z.H., (Zhifeng Huang) and X.C. conceptualized and designed the study. X.C. and B.L. wrote the first draft of the manuscript. Z.H. (Zilu Huang), X.W., D.H., and L.Y. carried out the experiments. X.C. and B.L. performed the data analysis. B.L., X.C., Z.H. (Zhifeng Huang) and C.Y. reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Lin, B., Huang, Z. et al. Umbilical cord blood platelet lysate preserves myofibroblast migration and mitigates hyperoxic lung injury. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04422-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-04422-1