Abstract
Background
Epinephrine is currently the only vasopressor recommended during neonatal cardiopulmonary resuscitation (CPR). Previous neonatal animal studies suggested intravenous (IV) vasopressin as a potential alternative; however, no neonatal study has compared the efficacy of intraosseous (IO) with IV vasopressin to epinephrine administration during CPR.
Design/Methods
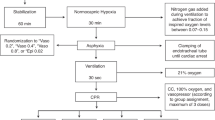
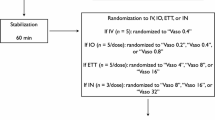
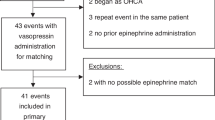
Newborn piglets (n = 8/group) were anesthetized, intubated, surgically instrumented, and exposed to normocapnic hypoxia followed by asphyxia and asystole. Piglets were resuscitated following randomization to 0.4IU/kg IV or IO vasopressin, or 0.02 mg/kg IV or IO epinephrine.
Results
There were no differences in median (IQR) time to return of spontaneous circulation (ROSC) between IV and IO vasopressin (254 (220-473) vs. 215 (200-240)sec, respectively, p = 0.143), IV and IO epinephrine (272 (265-278) vs. 233 (203-266)sec, respectively, p = 0.286), or all four groups (p = 0.312). The number of piglets that achieved ROSC was similar with IV and IO vasopressin (5 (63%) vs. 3 (38%), respectively, p = 0.619), IV and IO epinephrine (2 (25%) vs. 6 (75%), respectively, p = 0.132), and between all groups (p = 0.233).
Conclusions
Vasopressin given either via IO or IV had no difference in time to or rates of ROSC compared to IV and IO epinephrine.
Impact
-
This is the first neonatal animal study to compare intraosseous (IO) and intravenous (IV) vasopressin during cardiac arrest.
-
Time to and rates of return of spontaneous circulation (ROSC) were similar between IO and IV vasopressin.
-
Time to and incidence of ROSC were not statistically different between vasopressin and epinephrine, regardless of IV or IO administration.
-
Hemodynamic changes during cardiopulmonary resuscitation are similar between vasopressin and epinephrine groups.
-
Plasma vasopressor concentrations were similar between IO and IV routes
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Aziz, K. et al. Part 5: neonatal resuscitation: 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 142, S524–S550 (2020).
Wyckoff, M. H. et al. Neonatal life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 142, S185–S221 (2020).
Turner, D. W., Attridge, R. L. & Hughes, D. W. Vasopressin associated with an increase in return of spontaneous circulation in acidotic cardiopulmonary arrest patients. Ann. Pharmacother. 48, 986–991 (2014).
Papastylianou, A. & Mentzelopoulos, S. Current pharmacological advances in the treatment of cardiac arrest. Emerg. Med. Int. 2012, 815857 (2012).
Pelletier, J. S. et al. Low-dose vasopressin improves cardiac function in newborn piglets with acute hypoxia-reoxygenation. Shock 40, 320–326 (2013).
Pinto, M. et al. Evidence on adrenaline use in resuscitation and its relevance to newborn infants: a non-systematic review. Neonatology 111, 37–44 (2017).
Ramsie, M. et al. Cardiac agents during neonatal cardiopulmonary resuscitation. Neonatology 1–10 https://doi.org/10.1159/000535502 (2024).
O’Reilly, M. & Schmölzer, G. M. Evidence for vasopressors during cardiopulmonary resuscitation in newborn infants. Minerva Pediatr. https://doi.org/10.23736/s0026-4946.18.05452-x (2018).
Wenzel, V. et al. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N. Engl. J. Med. 350, 105–113 (2004).
O’Reilly, M., Lee, T. F., Cheung, P. Y. & Schmölzer, G. M. Vasopressin versus epinephrine during neonatal cardiopulmonary resuscitation of asphyxiated post-transitional piglets. Resusc. Plus 15, 100427 (2023).
Ramsie, M., Cheung, P. Y., Lee, T. F., O’Reilly, M., & Schmölzer, G. M. Comparison of various vasopressin doses to epinephrine during cardiopulmonary resuscitation in asphyxiated neonatal piglets. Pediatr. Res. 1–8 https://doi.org/10.1038/s41390-023-02858-x (2023).
Chaudhry, A. et al. Effect of vasopressin on brain and cardiac tissue during neonatal cardiopulmonary resuscitation of asphyxiated post-transitional piglets. Resusc. Plus 21, 100837 (2025).
Rawat, M. et al. Masked randomized trial of epinephrine versus vasopressin in an ovine model of perinatal cardiac arrest. Children 10, 349 (2023).
McNamara, P. J., Engelberts, D., Finelli, M., Adeli, K. & Kavanagh, B. P. Vasopressin improves survival compared with epinephrine in a neonatal piglet model of asphyxial cardiac arrest. Pediatr. Res. 75, 738–748 (2014).
Isayama, T. et al. The route, dose, and interval of epinephrine for neonatal resuscitation: a systematic review. Pediatrics 146, e20200586 (2020).
Schwindt, E. et al. Intraosseous access in neonates is feasible and safe–an analysis of a prospective nationwide surveillance study in Germany. Front. Pediatr. 10, 952632 (2022).
Ramsie, M., Cheung, P. Y., O’Reilly, M., Lee, T. F. & Schmölzer, G. M. Pharmacokinetic and pharmacodynamic evaluation of various vasopressin doses and routes of administration in a neonatal piglet model. Sci. Rep. 14, 23096 (2024).
Wenzel, V. et al. Intraosseous vasopressin improves coronary perfusion pressure rapidly during cardiopulmonary resuscitation in pigs. Crit. Care Med. 27, 1565–1569 (1999).
Fulkerson, J. et al. Effects of intraosseous tibial vs. intravenous vasopressin in a hypovolemic cardiac arrest model. West. J. Emerg. Med. 17, 222–228 (2016).
Johnson, D. et al. Effects of tibial intraosseous and IV administration of vasopressin on kinetics and survivability in cardiac arrest. Am. J. Emerg. Med. 34, 429–432 (2016).
Couper, K. et al. A randomized trial of drug route in out-of-hospital cardiac arrest. N. Engl. J. Med. 392, 336–348 (2025).
Sert et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Exp. Physiol. 105, 1459–1466 (2020).
Link, M. S. et al. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation S444–S464. https://doi.org/10.1161/cir.0000000000000261 (2015).
Schmölzer, G. M. et al. Cardiopulmonary resuscitation with chest compressions during sustained inflations. Circulation 128, 2495–2503 (2013).
Cheung, P. Y., Gill, R. S., & Bigam, D. L. A swine model of neonatal asphyxia. J. Visual. Exp. https://doi.org/10.3791/3166 (2011).
Pichler, G. et al. Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J. Pediatr. 163, 1558–1563 (2013).
Schmölzer, G. M. Chest compressions during sustained inflation during cardiopulmonary resuscitation in newborn infants translating evidence from animal studies to the bedside. JACC: Basic Transl. Sci. 4, 116–121 (2019).
Koo, J. et al. Chest compression superimposed with sustained inflation or 3:1 compression/ventilation ratio during neonatal cardiopulmonary resuscitation in the delivery room: a systematic review and meta-analysis. Children 12, 230 (2025).
Bruckner, M. et al. Haemodynamic changes with varying chest compression rates in asphyxiated piglets. Arch. Dis. Child Fetal Neonatal Ed. 108, 200–203 (2023).
Bruckner, M., O’Reilly, M., Lee, T. F., Cheung, P. Y. & Schmölzer, G. M. Chest compression rates of 60/min versus 90/min during neonatal cardiopulmonary resuscitation: a randomized controlled animal trial. Front. Pediatr. 11, 1214513 (2023).
Bruckner, M. et al. Chest compression rates of 90/min versus 180/min during neonatal cardiopulmonary resuscitation: a randomized controlled animal trial. Children 9, 1838 (2022).
Bruckner, M. et al. Effects of varying chest compression depths on carotid blood flow and blood pressure in asphyxiated piglets. Arch. Dis. Child Fetal Neonatal Ed. 106, 553–556 (2021).
Bruckner, M. et al. Assessment of optimal chest compression depth during neonatal cardiopulmonary resuscitation: a randomised controlled animal trial. Arch. Dis. Child Fetal Neonatal Ed. 107, 262–268 (2022).
O’Reilly, M., Lee, T. F., Cheung, P. Y., & Schmölzer, G. M. Comparison of hemodynamic effects of chest compression delivered via machine or human in asphyxiated piglets. Pediatr. Res. 1–4 https://doi.org/10.1038/s41390-023-02827-4 (2023).
Rajani, A. K., Chitkara, R., Oehlert, J. W. & Halamek, L. P. Comparison of umbilical venous and intraosseous access during simulated neonatal resuscitation. Pediatrics 128, e954–e958 (2011).
Haase, B., Springer, L. & Poets, C. F. Evaluating practioners’ preferences regarding vascular emergency access in newborn infants in the delivery room: a national survey. BMC Pediatr. 20, 405 (2020).
Ramsie, M., Cheung, P. Y., Law, B. & Schmölzer, G. M. Vasopressin versus epinephrine during cardiopulmonary resuscitation of asphyxiated newborns: a study protocol for a prospective, cluster, open label, single-center, randomized controlled phase 2 trial – The VERSE-Trial. Resusc. Plus 16, 100459 (2023).
Rubertsson, S., Nozari, A., & Wiklund, L. Cerebral blood flow and oxygenation with vasopressin or epinephrine during and after experimental CPR. Crit. Care Med. 27, 108A (1999).
Nozari, A., Rubertsson, S. & Wiklund, L. Differences in the pharmacodynamics of epinephrine and vasopressin during and after experimental cardiopulmonary resuscitation. Resuscitation 49, 59–72 (2001).
Mendler, M. R. et al. Successful resuscitation in a model of asphyxia and hemorrhage to test different volume resuscitation strategies. A study in newborn piglets after transition. Front. Pediatr. 6, 192 (2018).
Lascarrou, J. B. et al. Dysnatremia at ICU admission and functional outcome of cardiac arrest: insights from four randomised controlled trials. Crit. Care 27, 472 (2023).
Patel, K. et al. Vasopressin induced hyponatremia in infants <3 months of age in the neonatal intensive care unit. Front. Pediatr. 12, 1465785 (2024).
Schwindt, E. M. et al. Duration to establish an emergency vascular access and how to accelerate it: a simulation-based study performed in real-life neonatal resuscitation rooms. Pediatr. Crit. Care Med. 19, 468–476 (2018).
Ellemunter, H., Simma, B., Trawoger, R. & Maurer, H. Intraosseous lines in preterm and full term neonates. Arch. Dis. Child. Fetal Neonatal Ed. 80, F74–F75 (1999).
Mileder, L. P., Urlesberger, B. & Schwaberger, B. Use of intraosseous vascular access during neonatal resuscitation at a tertiary center. Front. Pediatr. 8, 20–25 (2020).
Engle, W. A. Intraosseous access for administration of medications in neonates. Clin. Perinatol. 33, 161–168 (2006).
Koo, J. et al. Chest compressions superimposed with sustained inflation during neonatal cardiopulmonary resuscitation: are we ready for a clinical trial? Arch. Dis. Child Fetal Neonatal Ed. 2024:110;2–7.
Schmölzer, G. M., O’Reilly, M., Fray, C., van Os, S. & Cheung, P. Y. Chest compression during sustained inflation versus 3:1 chest compression: ventilation ratio during neonatal cardiopulmonary resuscitation: a randomised feasibility trial. Arch. Dis. Child Fetal Neonatal Ed. 103, F455–F460 (2018).
Schmölzer G. M., et al. Sustained inflation and chest compression versus 3:1 chest compression to ventilation ratio during cardiopulmonary resuscitation of asphyxiated newborns (SURV1VE): a cluster randomised controlled trial. Arch Dis. Child Fetal Neonatal Ed. https://doi.org/10.1136/archdischild-2023-326383 (2023).
Funding
We would like to thank the public for donating money to our funding agencies: M.R. is a recipient of the Motyl Graduate Studentships in Cardiac Sciences, Women and Children’s Health Research Institute Graduate Studentship, and Alberta Excellence Graduate Scholarship. This study was supported by a Heart and Stroke Foundation of Canada Grant in Aid (G-22-0031980).
Author information
Authors and Affiliations
Contributions
Conception and design: G.M.S., P.Y.C., M.R., M.O.R., and T.F.L. Collection and assembly of data: G.M.S., P.Y.C., M.R., M.O.R., T.F.L., and R.H. Analysis and interpretation of the data: G.M.S., P.Y.C., M.R., M.O.R., T.F.L., and RH. Drafting of the 1st draft: M.R. Drafting of the article: G.M.S., P.Y.C., M.R., M.O.R., T.F.L., and R.H. Critical revision of the article for important intellectual content: G.M.S., P.Y.C., M.R., M.O.R., T.F.L., and R.H. Final approval of the article: G.M.S., P.Y.C., M.R., M.O.R., T.F.L., and R.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Patient consent was not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramsie, M., Cheung, PY., Hyderi, R. et al. Comparison of intravenous and intraosseous administration of vasopressin and epinephrine during cardiopulmonary resuscitation of asphyxiated neonatal piglets. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04423-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04423-0