Abstract
Background
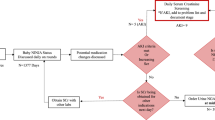
Early identification of kidney recovery in critically ill children and young adults undergoing continuous kidney support therapy (CKST) is essential to optimize care and minimize complications. Urinary neutrophil gelatinase-associated lipocalin (uNGAL) is a biomarker of acute kidney injury, but its utility in predicting CKST duration and guiding timely liberation remains unclear.
Methods
We retrospectively analyzed urinary uNGAL levels in pediatric intensive care unit (PICU) patients (aged 2 months to 25 years) who received CKST between July 2018 and April 2024. We evaluated two outcomes: (1) the ability of peak uNGAL levels during the first four days of CKST to predict prolonged therapy (>7 days), and (2) the performance of uNGAL measured during KST liberation attempts in predicting successful liberation.
Results
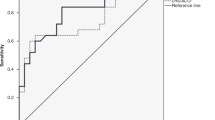
Among 57 patients, early peak uNGAL predicted CKST duration >7 days with good accuracy (AUC-ROC 0.85 [95% CI, 0.73–0.97], optimal cutoff 2600 ng/mL). uNGAL also showed excellent performance in predicting successful KST liberation (AUC-ROC 0.95 [95% CI, 0.89–1.00], optimal cutoff 900 ng/mL).
Conclusions
uNGAL may be useful for predicting prolonged CKST duration and guiding KST liberation in critically ill children. Larger prospective studies are needed to confirm its role in personalized CKST management.
Impact statement
-
Peak uNGAL levels within the first four days of CKST are strongly associated with treatment duration >7 days, showing good predictive accuracy.
-
uNGAL demonstrates excellent performance in identifying patients likely to achieve successful KST liberation.
-
uNGAL measured during KST may support clinical decision-making by providing timing insights, potentially optimizing treatment duration in critically ill pediatric patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alobaidi, R. et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. 172, 257–268 (2018).
Gist, K. M. et al. Time to continuous renal replacement therapy initiation and 90-day major adverse kidney events in children and young adults. JAMA Netw. Open 7, e2349871 (2024).
Katulka, R. J. et al. Determining the optimal time for liberation from renal replacement therapy in critically ill patients: a systematic review and meta-analysis (DOnE RRT). Crit. Care 24, 50 (2020).
Yoshida, T. et al. Different roles of functional and structural renal markers measured at discontinuation of renal replacement therapy for acute kidney injury. Blood Purif. 52, 786–792 (2023).
Komaru, Y. et al. Urinary neutrophil gelatinase-associated lipocalin and plasma IL-6 in discontinuation of continuous venovenous hemodiafiltration for severe acute kidney injury: a multicenter prospective observational study. Ann. Intensive Care 13, 42 (2023).
von Groote, T. et al. Proenkephalin A 119–159 predicts early and successful liberation from renal replacement therapy in critically ill patients with acute kidney injury: a post hoc analysis of the ELAIN trial. Crit. Care 26, 333 (2022).
von Groote, T. et al. Evaluation of Proenkephalin A 119–159 for liberation from renal replacement therapy: an external, multicenter pilot study in critically ill patients with acute kidney injury. Crit. Care 27, 276 (2023).
Hoste, E. et al. Identification and validation of biomarkers of persistent acute kidney injury: the RUBY study. Intensive Care Med 46, 943–953 (2020).
Bagshaw, S. M. et al. External validation of urinary C–C motif chemokine ligand 14 (CCL14) for prediction of persistent acute kidney injury. Crit. Care 25, 185 (2021).
Chen, Y. T. et al. Performance of urinary C–C motif chemokine ligand 14 for the prediction of persistent acute kidney injury: a systematic review and meta-analysis. Crit. Care 27, 318 (2023).
Meersch, M. et al. Predicting the development of renal replacement therapy indications by combining the furosemide stress test and chemokine (C-C Motif) ligand 14 in a cohort of postsurgical patients. Crit. Care Med. 51, 1033–1042 (2023).
Stenson, E. K. et al. Factors associated with successful liberation from continuous renal replacement therapy in children and young adults: analysis of the worldwide exploration of renal replacement outcomes collaborative in Kidney Disease Registry. Intensive Care Med 50, 861–872 (2024).
Daverio, M. et al. Continuous kidney replacement therapy practices in pediatric intensive care units across Europe. JAMA Netw. Open 5, E2246901 (2022).
Haga, T., Ide, K. & Tani, M. Characteristics of pediatric continuous renal replacement therapies in hospitals with pediatric intensive care units in Japan. Ther. Apheresis Dial. 27, 562–570 (2023).
Fuhrman, D. Y., Gist, K. M. & Akcan-Arikan, A. Current practices in pediatric continuous kidney replacement therapy: a systematic review-guided multinational modified Delphi consensus study. Pediatr. Nephrol. 38, 2817–2826 (2023).
Gist, K. M. et al. Continuous renal replacement therapy: current state and future directions for worldwide practice. Pediatr. Crit. Care Med. 25, 554–560 (2024).
Goldstein, S. L. et al. Consensus-based recommendations on priority activities to address acute kidney injury in children: a modified Delphi Consensus statement. JAMA Netw. Open 5, E2229442 (2022).
Goldstein, S. L. et al. Real-time acute kidney injury risk stratification–biomarker directed fluid management improves outcomes in critically ill children and young adults. Kidney Int. Rep. 8, 2690–2700 (2023).
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 61, 344–349 (2008).
Schoenfeld, D. A. & Bernard, G. R. ARDS Network. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit. Care Med. 30, 1772–1777 (2002).
Billings Iv F. T. & Shaw A. D. Clinical trial endpoints in acute kidney injury. in Nephron - Clinical Practice. Vol 127 (S. Karger AG, 2014) 89–93. https://doi.org/10.1159/000363725
Kellum, J. A. et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2, 1–138 (2012).
Fuhrman, D. Y. et al. Major adverse kidney events in pediatric continuous kidney replacement therapy. JAMA Netw. Open 7, E240243 (2024).
Starr, M. C. et al. Continuous kidney replacement therapy and survival in children and young adults: findings from the multinational WE-ROCK collaborative. Am. J. Kidney Dis. 84, 406–415.e1 (2024).
Paragas, N. et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat. Med. 17, 216–222 (2011).
Author information
Authors and Affiliations
Contributions
G.C., I.C.B., K.A.K., and A.C.N. collected the clinical data. G.C. drafted the manuscript, while G.C. and S.L.G. conducted the data analysis and interpretation, serving as the primary authors. K.M.G., I.C.B., A.C.N., and S.L.G. contributed to the critical revision and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
SLG reports receiving personal fees from Baxter Healthcare, BioPorto Diagnostics, Calcimedica, Fresenius, Novartis, Nuwellis, MediBeacon, Medtronic, Otsuka and SeaStar Medical. KMG reports receiving funding from the Gerber Foundation for the MINI-ROCKET study and is a consultant for Bioporto Diagnostics. She is a member of the Medical Advisory Board for SeaStar Medical and received speaker honoraria from Fresenius. None of these entities had any input into the study or manuscript, and these relationships are not relevant to the manuscript. No other disclosures were reported.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ceschia, G., Gist, K.M., Clover-Brown, I. et al. Urine neutrophil gelatinase-associated lipocalin predicts kidney support therapy duration and liberation in critically ill children. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04430-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-04430-1