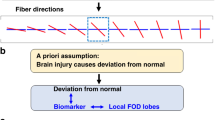
Abstract
Neonatal encephalopathy (NE) is a significant global health concern. It is a leading cause of long-term neurodevelopmental impairment, with hypoxic-ischaemic perinatal brain injury being the most common underlying contributor. Although therapeutic hypothermia has reduced mortality and improved outcomes for some affected infants, many survivors experience neurodevelopmental disability, including cerebral palsy and/or deficits in cognition, behaviour, and executive functioning. Early and accurate prognostication and identification of injury severity remain a challenge due to evolving clinical signs and multiple etiologies. Magnetic resonance imaging (MRI) is the gold standard for characterizing NE-related brain injury. Diffusion-weighted imaging (DWI) enables early detection of injury, and proton magnetic resonance spectroscopy (1H-MRS), specifically the Lac/NAA peak area ratio from basal ganglia and thalamus, provides robust prognostic indicators of two-year neurodevelopmental outcomes. MRI scoring systems incorporating multiple modalities correlate well with later neurodevelopmental outcomes. Advanced imaging modalities, such as diffusion tensor imaging (DTI), arterial spin labelling (ASL), and blood oxygen level-dependent (BOLD) imaging, offer further insights into microstructural integrity, perfusion, and functional connectivity. By standardizing acquisition protocols and post-processing, MRI biomarkers can serve as reliable, early surrogate endpoints in neuroprotection trials, allowing smaller sample sizes and accelerating clinical translation. MRI and 1H-MRS integration enhances prognostication, guides clinical management, and supports informed decision-making in NE care.
Impact
-
This article highlights the importance of state-of-the-art MRI and MRS techniques for assessing neonatal encephalopathy (NE), emphasizing optimized protocols, accurate interpretation, and the use of MRI scoring systems to enhance clinical decision-making. It provides a comprehensive guide to advanced MRI/MRS acquisition and interpretation in neonates with NE, addressing current limitations and future directions. By optimizing neonatal MRI/MRS practices, this work aims to improve early diagnosis and prognostication, guide treatment strategies, and ultimately improve the management of neonates with NE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
McIntyre, S. et al. Neonatal encephalopathy: focus on epidemiology and underexplored aspects of etiology. Semin Fetal Neonatal Med. 26, 101265 (2021).
Collaborators, G. B. D. N. S. D. Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 23, 344–381 (2024).
Badawi, N. et al. Antepartum risk factors for newborn encephalopathy: The Western Australian Case-Control Study. BMJ 317, 1549–1553 (1998).
Badawi, N., Kurinczuk, J. J., Blair, E., Keogh, J. & Stanley, F. Early prediction of the development of microcephaly after hypoxic-ischemic encephalopathy in the full-term newborn. Pediatrics 97, 151–152 (1996).
American College of Obstetricians and Gynecologist Executive Summary Neonatal Encephalopathy and Neurologic Outcome, Second Edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet. Gynecol. 123, 896–901 (2014).
Cowan, F. et al. Origin and Timing of Brain Lesions in Term Infants with Neonatal Encephalopathy. Lancet 361, 736–742 (2003).
Branagan, A. et al. Consensus definition and diagnostic criteria for neonatal encephalopathy-study protocol for a real-time modified Delphi study. Pediatr. Res. (2024).
Lorek, A. et al. Delayed (“Secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr. Res. 36, 699–706 (1994).
Azzopardi, D. et al. Prognosis of newborn infants with hypoxic-ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr. Res. 25, 445–451 (1989).
Roth, S. C. et al. Relation between cerebral oxidative metabolism following birth asphyxia, and neurodevelopmental outcome and brain growth at one year. Dev. Med. Child Neurol. 34, 285–295 (1992).
Cady, E. B. et al. Non-invasive investigation of cerebral metabolism in newborn infants by phosphorus nuclear magnetic resonance spectroscopy. Lancet 1, 1059–1062 (1983).
Hope, P. L. et al. Cerebral energy metabolism studied with phosphorus NMR spectroscopy in normal and birth-asphyxiated infants. Lancet 2, 366–370 (1984).
Iwata, O. et al. Supra- and sub-baseline phosphocreatine recovery in developing brain after transient hypoxia-ischaemia: relation to baseline energetics, insult severity and outcome. Brain 131, 2220–2226 (2008).
Schreglmann, M., Ground, A., Vollmer, B. & Johnson, M. J. Systematic review: long-term cognitive and behavioural outcomes of neonatal hypoxic-ischaemic encephalopathy in children without cerebral palsy. Acta Paediatr. 109, 20–30 (2020).
Spencer, A. P. C. et al. Disrupted brain connectivity in children treated with therapeutic hypothermia for neonatal encephalopathy. NeuroImage: Clin. 30 (2021).
Groenendaal, F. & de Vries, L. S. Fifty years of brain imaging in neonatal encephalopathy following perinatal Asphyxia. Pediatr. Res. 81, 150–155 (2017).
Barkovich, A. J. et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR Scoring Systems. AJNR Am. J. Neuroradiol. 19, 143–149 (1998).
Rutherford, M. A. et al. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 102, 323–328 (1998).
Weeke, L. C. et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J. Pediatr. 192, 33–40.e32 (2018).
Weeke, L. C., Groenendaal, F. & de Vries, L. S. MRI scoring systems for long-term outcome prediction in neonatal encephalopathy due to hypoxia-ischemia: in search of the crystal ball. Pediatr Res. (2024).
Parmentier, C. E. J., de Vries, L. S. & Groenendaal, F. Magnetic Resonance Imaging in (near-)Term Infants with Hypoxic-Ischemic Encephalopathy. Diagnostics 12, 645 (2022).
Miller, S. P. et al. Patterns of brain injury in term neonatal encephalopathy. J. Pediatr. 146, 453–460 (2005).
Martinez-Biarge, M., Diez-Sebastian, J., Rutherford, M. A. & Cowan, F. M. Outcomes after central grey matter injury in term perinatal hypoxic-ischaemic encephalopathy. Early Hum. Dev. 86, 675–682 (2010).
Shankaran, S. et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch. Dis. Child Fetal Neonatal Ed. 97, F398–F404 (2012).
Garvey, A. A., El-Shibiny, H., Yang, E., Inder, T. E. & El-Dib, M. Differences between early and late MRI in infants with neonatal encephalopathy following therapeutic hypothermia. Pediatr. Res. 94, 1011–1017 (2023).
Langeslag, J. F. et al. Outcome prediction and inter-rater comparison of four brain magnetic resonance imaging scoring systems of infants with perinatal asphyxia and therapeutic hypothermia. Neonatology 119, 311–319 (2022).
Wendland, M. F. et al. Early diffusion-weighted MRI as a predictor of Caspase-3 activation after hypoxic-ischemic insult in neonatal rodents. Stroke 39, 1862–1868 (2008).
Calabrese, E. et al. Correlating quantitative MRI-based apparent diffusion coefficient metrics with 24-month neurodevelopmental outcomes in neonates from the heal trial. Radiology 308, e223262 (2023).
Barkovich, A. J. et al. MR Imaging, MR Spectroscopy, And Diffusion Tensor Imaging Of Sequential Studies In Neonates With Encephalopathy. AJNR Am. J. Neuroradiol. 27, 533–547 (2006).
McKinstry, R. C. et al. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 59, 824–833 (2002).
Bednarek, N. et al. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 78, 1420–1427 (2012).
Cihangiroglu, M. et al. The utility of high B-value DWI in evaluation of ischemic stroke at 3T. Eur. J. Radio. 78, 75–81 (2011).
Machie, M., de Vries, L. S. & Inder, T. Advances in neuroimaging biomarkers and scoring. Clin. Perinatol. 51, 629–647 (2024).
Hung, S. C., Tu, Y. F., Hunter, S. E. & Guimaraes, C. MRI predictors of long-term outcomes of neonatal hypoxic ischaemic encephalopathy: a primer for radiologists. Br. J. Radio. 97, 1067–1077 (2024).
Ouwehand, S. et al. Predictors of outcomes in hypoxic-ischemic encephalopathy following hypothermia: a meta-analysis. Neonatology 117, 411–427 (2020).
Brissaud, O. et al. Efficiency of fractional anisotropy and apparent diffusion coefficient on diffusion tensor imaging in prognosis of neonates with hypoxic-ischemic encephalopathy: a methodologic prospective pilot study. AJNR Am. J. Neuroradiol. 31, 282–287 (2010).
Tusor, N. et al. Prediction of neurodevelopmental outcome after hypoxic-ischemic encephalopathy treated with hypothermia by diffusion tensor imaging analyzed using tract-based spatial statistics. Pediatr. Res. 72, 63–69 (2012).
Thayyil, S. et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 125, e382–e395 (2010).
Mitra, S. et al. Proton magnetic resonance spectroscopy lactate/N-Acetylaspartate within 2 weeks of birth accurately predicts 2-year motor, cognitive and language outcomes in neonatal encephalopathy after therapeutic hypothermia. Arch. Dis. Child Fetal Neonatal Ed. 104, F424–F432 (2019).
Lally, P. J. et al. Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: a prospective multicentre cohort study. Lancet Neurol. 18, 35–45 (2019).
Azzopardi, D. et al. Prospective qualification of early cerebral biomarkers in a randomised trial of treatment with xenon combined with moderate hypothermia after birth asphyxia. EBioMedicine 47, 484–491 (2019).
Robertson, N. J. et al. Cerebral intracellular lactic alkalosis persisting months after neonatal encephalopathy measured by magnetic resonance spectroscopy. Pediatr. Res. 46, 287–296 (1999).
Azzopardi, D. et al. Moderate hypothermia within 6 H of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-XE): a proof-of-concept, open-label, randomised controlled Trial. Lancet Neurol. 15, 145–153 (2016).
Pang, R. et al. Proton magnetic resonance spectroscopy lactate/N-Acetylaspartate within 48 H predicts cell death following varied neuroprotective interventions in a piglet model of hypoxia-ischemia with and without inflammation-sensitization. Front Neurol. 11, 883 (2020).
Kyng, K. J. et al. Short-term outcomes of remote ischemic postconditioning 1 H after perinatal hypoxia-ischemia in term piglets. Pediatr. Res. 89, 150–156 (2021).
Thayyil, S. et al. Whole-body hypothermia, cerebral magnetic resonance biomarkers, and outcomes in neonates with moderate or severe hypoxic-ischemic encephalopathy born at tertiary care centers vs other facilities: a nested study within a randomized clinical trial. JAMA Netw. Open 6, e2312152 (2023).
Wu, T. W. et al. Cerebral lactate concentration in neonatal hypoxic-ischemic encephalopathy: in relation to time, characteristic of injury, and serum lactate concentration. Front. Neurol. 9, 293 (2018).
Barta, H. et al. Predictive performance and metabolite dynamics of proton MR spectroscopy in neonatal hypoxic-ischemic encephalopathy. Pediatr. Res. 91, 581–589 (2022).
Shibasaki, J. et al. Changes in brain metabolite concentrations after neonatal hypoxic-ischemic encephalopathy. Radiology 288, 840–848 (2018).
Robertson, N. J. et al. Early increases in brain myo-inositol measured by proton magnetic resonance spectroscopy in term infants with neonatal encephalopathy. Pediatr. Res. 50, 692–700 (2001).
Lange, T. et al. Pitfalls in lactate measurements at 3t. AJNR Am. J. Neuroradiol. 27, 895–901 (2006).
Roelants-Van Rijn, A. M., van der Grond, J., de Vries, L. S. & Groenendaal, F. Value of (1)H-MRS Using Different Echo Times in Neonates with Cerebral Hypoxia-Ischemia. Pediatr. Res. 49, 356–362 (2001).
Choi, C. et al. Proton spectral editing for discrimination of Lactate and Threonine 1.31 PPM resonances in human brain in vivo. Magn. Reson. Med. 56, 660–665 (2006).
Moss, H. G., Jenkins, D. D., Yazdani, M. & Brown, T. R. Identifying the translational complexity of magnetic resonance spectroscopy in neonates and infants. NMR Biomed. 32, e4089 (2019).
Thayyil, S. et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob. Health 9, e1273–e1285 (2021).
Wilson, M. Spant: An R Package for magnetic resonance spectroscopy analysis. J. Open Source Softw. 6 (2021).
Provencher, S. W. Estimation of metabolite concentrations from localized in vivo proton NMR Spectra. Magn. Reson Med. 30, 672–679 (1993).
Montaldo, P. et al. Whole-body hypothermia vs targeted normothermia for neonates with mild encephalopathy: a multicenter pilot randomized clinical trial. JAMA Netw. Open 7, e249119 (2024).
Detre, J. A., Rao, H., Wang, D. J., Chen, Y. F. & Wang, Z. Applications of arterial spin labeled MRI in the brain. J. Magn. Reson Imaging 35, 1026–1037 (2012).
Proisy, M. et al. Changes in brain perfusion in successive arterial spin labeling MRI scans in neonates with hypoxic-ischemic encephalopathy. Neuroimage Clin. 24, 101939 (2019).
Tierradentro-Garcia, L. O. et al. Cerebral blood flow of the neonatal brain after hypoxic-ischemic injury. Am. J. Perinatol. 40, 475–488 (2023).
Wintermark, P. et al. Brain perfusion in asphyxiated newborns treated with therapeutic hypothermia. AJNR Am. J. Neuroradiol. 32, 2023–2029 (2011).
Wintermark, P., Hansen, A., Warfield, S. K., Dukhovny, D. & Soul, J. S. Near-infrared spectroscopy versus magnetic resonance imaging to study brain perfusion in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neuroimage 85, 287–293 (2014).
Grant, P. E. & Yu, D. Acute injury to the immature brain with hypoxia with or without hypoperfusion. Radio. Clin. North Am. 44, 63–77 (2006).
De Vis, J. B. et al. Arterial spin-labelling perfusion mri and outcome in neonates with hypoxic-ischemic encephalopathy. Eur. Radio. 25, 113–121 (2015).
Tuura, R. O. et al. Elevated cerebral perfusion in neonatal encephalopathy is associated with neurodevelopmental impairments. Pediatr Res. (2024).
Zheng, Q. et al. Cerebral pulsed arterial spin labeling perfusion weighted imaging predicts language and motor outcomes in neonatal hypoxic-ischemic encephalopathy. Front Pediatr. 8, 576489 (2020).
Tang, S. et al. Application of a 3D pseudocontinuous arterial spin-labeled perfusion MRI scan combined with a postlabeling delay value in the diagnosis of neonatal hypoxic-ischemic encephalopathy. PLoS One 14, e0219284 (2019).
Wintermark, P., Moessinger, A. C., Gudinchet, F. & Meuli, R. Temporal evolution of MR perfusion in neonatal hypoxic-ischemic encephalopathy. J. Magn. Reson. Imaging 27, 1229–1234 (2008).
Massaro, A. N. et al. Brain perfusion in encephalopathic newborns after therapeutic hypothermia. AJNR Am. J. Neuroradiol. 34, 1649–1655 (2013).
Boerwinkle, V. L. et al. Resting-state functional magnetic resonance imaging network association with mortality, epilepsy, cognition, and motor two-year outcomes in suspected severe neonatal acute brain injury. Pediatr. Neurol. 152, 41–55 (2024).
Li, H. X. et al. Resting-state network complexity and magnitude changes in neonates with severe hypoxic ischemic encephalopathy. Neural Regen. Res. 14, 642–648 (2019).
Merhar, S. L. et al. Neonatal functional and structural connectivity are associated with cerebral palsy at two years of age. Am. J. Perinatol. 37, 137–145 (2020).
Pinto, C. R. et al. The Role of Early Functional Neuroimaging in Predicting Neurodevelopmental Outcomes in Neonatal Encephalopathy. Eur. J. Pediatr. 182, 1191–1200 (2023).
Schmithorst, V. J. et al. Evidence that neurovascular coupling underlying the bold effect increases with age during childhood. Hum. Brain Mapp. 36, 1–15 (2015).
Gunn, A. J. & Battin, M. Towards faster studies of neonatal encephalopathy. Lancet Neurol. 18, 21–22 (2019).
Burgod, C. et al. Duration of birth depression and neurodevelopmental outcomes after whole-body hypothermia for hypoxic ischemic encephalopathy in India, Sri Lanka and Bangladesh - an Exploratory Analysis of the Helix Trial. Lancet Reg. Health Southeast Asia 20, 100284 (2024).
Wisnowski, J. L. et al. Integrating neuroimaging biomarkers into the multicentre, high-dose erythropoietin for asphyxia and encephalopathy (HEAL) trial: rationale, protocol and harmonisation. BMJ Open 11, e043852 (2021).
Wisnowski, J. L. et al. Brain injury outcomes after adjuvant erythropoietin neuroprotection for moderate or severe neonatal hypoxic-ischemic encephalopathy: a report from the heal trial. Dev. Neurosci. 46, 285–296 (2024).
Garegrat, R. et al. Whole-body hypothermia in mild neonatal encephalopathy: protocol for a multicentre Phase III randomised controlled trial. BMC Pediatr. 24, 460 (2024).
de Vries, L. S. & Groenendaal, F. Patterns of neonatal hypoxic-ischaemic brain injury. Neuroradiology 52, 555–566 (2010).
Rutherford, M. et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 9, 39–45 (2010).
Trivedi, S. B. et al. A validated clinical MRI injury scoring system in neonatal hypoxic-ischemic encephalopathy. Pediatr. Radio. 47, 1491–1499 (2017).
Shankaran, S. et al. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 167, 987–993 e983 (2015).
Ni Bhroin, M. et al. Relationship between MRI scoring systems and neurodevelopmental outcome at two years in infants with neonatal encephalopathy. Pediatr. Neurol. 126, 35–42 (2022).
Mohammad, K. et al. Consensus approach for standardization of the timing of brain magnetic resonance imaging and classification of brain injury in neonates with neonatal encephalopathy/hypoxic-ischemic encephalopathy: A Canadian perspective. Pediatr. Neurol. 166, 16–31 (2025).
Wisnowski, J. L. et al. Neuroimaging in the term newborn with neonatal encephalopathy. Semin. Fetal Neonatal Med. 26, 101304 (2021).
Kang, O. H. et al. Correlation of Different MRI scoring systems with long-term cognitive outcome in cooled asphyxiated newborns. Children 10, 1295 (2023).
Guarnera, A. et al. Predictive Value of MRI in hypoxic-ischemic encephalopathy treated with therapeutic hypothermia. Children 10 (2023).
van Rooij, L. G. et al. Effect of treatment of subclinical neonatal seizures detected with AEEG: randomized, controlled trial. Pediatrics 125, e358–e366 (2010).
Andorka, C. et al. The predictive value of MRI scores for neurodevelopmental outcome in infants with neonatal encephalopathy. Pediatr. Res. 97, 253–260 (2025).
Wu, Y. W. et al. How well does neonatal neuroimaging correlate with neurodevelopmental outcomes in infants with hypoxic-ischemic encephalopathy?. Pediatr. Res. 94, 1018–1025 (2023).
Molavi, M., Vann, S. D., de Vries, L. S., Groenendaal, F. & Lequin, M. Signal change in the mammillary bodies after perinatal asphyxia. AJNR Am. J. Neuroradiol. 40, 1829–1834 (2019).
Annink, K. V. et al. Mammillary body atrophy and other MRI correlates of school-age outcome following neonatal hypoxic-ischemic encephalopathy. Sci. Rep. 11, 5017 (2021).
Gonzalez, F. F. et al. Perinatal arterial ischemic stroke diagnosed in infants receiving therapeutic hypothermia for hypoxic-ischemic encephalopathy. Pediatr. Res. 97, 1140–1146 (2025).
Boudes, E., Tan, X., Saint-Martin, C., Shevell, M. & Wintermark, P. MRI obtained during versus after hypothermia in asphyxiated newborns. Arch. Dis. Child Fetal Neonatal Ed. 100, F238–F242 (2015).
Cascio, A. et al. Discussing brain magnetic resonance imaging results for neonates with hypoxic-ischemic encephalopathy treated with hypothermia: a challenge for clinicians and parents. eNeurologicalSci 29, 100424 (2022).
Goergen, S. K. et al. Early MRI in term infants with perinatal hypoxic-ischaemic brain injury: interobserver agreement and MRI predictors of outcome at 2 years. Clin. Radio. 69, 72–81 (2014).
Lawrence, R. K. & Inder, T. E. Anatomic changes and imaging in assessing brain injury in the term infant. Clin. Perinatol. 35, 679–693 (2008).
Group, O. L. o. E. W. The Oxford 2011 Levels of Evidence. (2011).
Wu, T. W. et al. Maintenance of whole-body therapeutic hypothermia during patient transport and magnetic resonance imaging. Pediatr. Radio. 44, 613–617 (2014).
Thoresen, M. et al. MRI combined with early clinical variables are excellent outcome predictors for newborn infants undergoing therapeutic hypothermia after perinatal asphyxia. EClinicalMedicine 36, 100885 (2021).
Sharon, D. et al. Adequacy of an in-Neonatal Intensive Care Unit 1t magnetic resonance imaging compared with 3T magnetic resonance imaging for clinical management. Pediatr. Neurol. 161, 34–39 (2024).
Singh, E. et al. Improving access to magnetic resonance imaging for the newborn. J. Neonatal Nurs. 29, 199–202 (2023).
Roychaudhuri, S., Ersen, Y., El-Dib, M. & Inder, T. Point of care magnetic resonance neonatal neuroimaging applications and early imaging in infants under active therapeutic hypothermia: a perspective. J. Perinatol. 44, 1228–1232 (2024).
Abate, F. et al. Unity: a low-field magnetic resonance neuroimaging initiative to characterize neurodevelopment in low and middle-income settings. Dev. Cogn. Neurosci. 69, 101397 (2024).
Lew, C. O. et al. Artificial intelligence outcome prediction in neonates with encephalopathy (AI-Opine). Radiol. Artif. Intell. 6, e240076 (2024).
Sullivan, B. A. et al. Transforming neonatal care with artificial intelligence: challenges, ethical consideration, and opportunities. J. Perinatol. 44, 1–11 (2024).
Lewis, J. D. et al. Automated neuroprognostication via machine learning in neonates with hypoxic-ischemic encephalopathy. Ann. Neurol. (2024).
Ouyang, M., Whitehead, M. T., Mohapatra, S., Zhu, T. & Huang, H. Machine-learning based prediction of future outcome using multimodal mri during early childhood. Semin Fetal Neonatal Med. 29, 101561 (2024).
Peeples, E. S. et al. Predictive models of neurodevelopmental outcomes after neonatal hypoxic-ischemic encephalopathy. Pediatrics 147 (2021).
Alderliesten, T. et al. MRI and Spectroscopy in (near) term neonates with perinatal asphyxia and therapeutic hypothermia. Arch. Dis. Child Fetal Neonatal Ed. 102, F147–F152 (2017).
Garegrat, R. et al. Early and extended erythropoietin monotherapy after hypoxic ischaemic encephalopathy: a multicentre double-blind pilot randomised controlled trial. Arch. Dis. Child Fetal Neonatal Ed. 109, 594–601 (2024).
Lindner, T. et al. Current state and guidance on arterial spin labeling perfusion MRI in clinical neuroimaging. Magn. Reson Med. 89, 2024–2047 (2023).
Cheong, J. L. et al. Prognostic utility of magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy: substudy of a randomized trial. Arch. Pediatr. Adolesc. Med. 166, 634–640 (2012).
Zhang, H. et al. Age-specific optimization of T1-weighted brain Mri throughout infancy. Neuroimage 199, 387–395 (2019).
Acknowledgements
We would like to thank Paediatric Research for editorial support and Prof. Deirdre Murray for supporting the series as Chair of the Brain, Development, and Imaging section of the European Society of Paediatric Research.
Funding
No financial assistance was received in support of the study
Author information
Authors and Affiliations
Consortia
Contributions
This manuscript follows the Paediatric Research Author instructions for consortia formatting. All main authors have individually contributed to drafting the manuscript. All main authors and individually named consortia authors have revised the manuscript and approved the final version. Kasper Jacobsen Kyng and Ted Carl Kejlberg Andelius have conceptualized and designed the Magnetic Resonance Imaging and Spectroscopy in the Neonate series - European Society for Paediatric Research (ESPR). Kasper Jacobsen Kyng has edited and finalized the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Laptook, A., Garvey, A.A., Adams, C. et al. Magnetic resonance imaging and spectroscopy in neonatal encephalopathy: current consensus position and future opportunities. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04448-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04448-5
This article is cited by
-
Brain imaging as a predictor of neurodevelopmental outcomes in neonatal encephalopathy
Pediatric Research (2025)