Abstract
Background
Exercise is increasingly recognized as an effective strategy to improve cancer prevention and prognosis. Several biological mechanisms mediating these benefits have been proposed, but the role of epigenetics remains largely unknown. Since epigenetics is highly susceptible to lifestyle factors, we hypothesized that exercise could affect the epigenome landscape in cancer tissues.
Methods
Rats implanted with AT1 prostate tumors were randomized to either control or exercise training. microRNA expression, DNA methylation and histone acetylation were analyzed in the tumor tissue.
Results
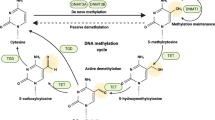
MiR-27a-5p appeared to be differently expressed between sedentary and trained rats. Furthermore, exercise increased global DNA methylation and decreased DNA methyltransferases mRNA expression in the tumor tissue. Histone acetylation however remained unaltered.
Conclusion
Overall, exercise might reverse some of the cancer-related epigenetic alterations in the prostate tumor tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018:68:394–424.
Jerónimo C, Bastian PJ, Bjartell A, Carbone GM, Catto JWF, Clark SJ, et al. Epigenetics in prostate cancer: biologic and clinical relevance. Eur Urol. 2011;60:753–66.
Kgatle MM, Kalla AA, Islam MM, Sathekge M, Moorad R. Prostate cancer: epigenetic alterations, risk factors, and therapy. Prostate Cancer. 2016;2016:5653862.
Ngollo M, Dagdemir A, Karsli-Ceppioglu S, Judes G, Pajon A, Penault-Llorca F, et al. Epigenetic modifications in prostate cancer. Epigenomics. 2014;6:415–26.
Isanejad A, Alizadeh AM, Amani Shalamzari S, Khodayari H, Khodayari S, Khori V, et al. MicroRNA-206, let-7a and microRNA-21 pathways involved in the anti-angiogenesis effects of the interval exercise training and hormone therapy in breast cancer. Life Sci. 2016;151:30–40.
Gueritat J, Lefeuvre-Orfila L, Vincent S, Cretual A, Ravanat J-L, Gratas-Delamarche A, et al. Exercise training combined with antioxidant supplementation prevents the antiproliferative activity of their single treatment in prostate cancer through inhibition of redox adaptation. Free Radic Biol Med. 2014;77:95–105.
Jackson BL, Grabowska A, Ratan HL. MicroRNA in prostate cancer: functional importance and potential as circulating biomarkers. BMC Cancer. 2014;14:1–10.
Barros-Silva D, Costa-Pinheiro P, Duarte H, Sousa EJ, Evangelista AF, Graça I, et al. MicroRNA-27a-5p regulation by promoter methylation and MYC signaling in prostate carcinogenesis. Cell Death Dis. 2018;9:1–15.
Khori V, Amani Shalamzari S, Isanejad A, Alizadeh AM, Alizadeh S, Khodayari S, et al. Effects of exercise training together with tamoxifen in reducing mammary tumor burden in mice: Possible underlying pathway of miR-21. Eur J Pharm. 2015;765:179–87.
Dufresne S, Rébillard A, Muti P, Friedenreich CM, Brenner DR. A review of physical activity and circulating miRNA expression: implications in cancer risk and progression. Cancer Epidemiol Biomark Prev. 2018;27:11–24.
Yegnasubramanian S, Haffner MC, Zhang Y, Gurel B, Cornish TC, Wu Z, et al. DNA hypomethylation arises later in prostate cancer progression than CpG island hypermethylation and contributes to metastatic tumor heterogeneity. Cancer Res. 2008;68:8954–67.
Zelic R, Fiano V, Grasso C, Zugna D, Pettersson A, Gillio-Tos A, et al. Global DNA hypomethylation in prostate cancer development and progression: a systematic review. Prostate Cancer Prostatic Dis. 2015;18:1–12.
Massie CE, Mills IG, Lynch AG. The importance of DNA methylation in prostate cancer development. J Steroid Biochem Mol Biol. 2017;166:1–15.
Perry AS, Watson RWG, Lawler M, Hollywood D. The epigenome as a therapeutic target in prostate cancer. Nat Rev Urol. 2010;7:668–80.
Yuasa Y, Nagasaki H, Akiyama Y, Hashimoto Y, Takizawa T, Kojima K, et al. DNA methylation status is inversely correlated with green tea intake and physical activity in gastric cancer patients. Int J Cancer. 2009;124:2677–82.
Dai JY, Wang B, Wang X, Cheng A, Kolb S, Stanford JL, et al. Vigorous physical activity is associated with lower risk of metastatic-lethal progression in prostate cancer and hypomethylation in the CRACR2A gene. Cancer Epidemiol Biomark Prev. 2019;28:258–64.
Weichert W, Röske A, Gekeler V, Beckers T, Stephan C, Jung K, et al. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer. 2008;98:604–10.
Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, et al. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262.
Zimmer P, Baumann FT, Bloch W, Schenk A, Koliamitra C, Jensen P, et al. Impact of exercise on pro inflammatory cytokine levels and epigenetic modulations of tumor-competitive lymphocytes in Non-Hodgkin-Lymphoma patients-randomized controlled trial. Eur J Haematol. 2014;93:527–32.
Zimmer P, Bloch W, Schenk A, Zopf EM, Hildebrandt U, Streckmann F, et al. Exercise-induced natural killer cell activation is driven by epigenetic modifications. Int J Sports Med. 2015;36:510–5.
Acknowledgements
The authors thank the team from the M2S lab and from the Emily Ho lab., the Ecole Normale Supérieure (ENS) Rennes, the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche (MENESR), the ARC Foundation for Cancer Research, and the Oregon Agricultural Experimental Station Hatch Funds (OR00735).
Funding
SD was supported by the Ecole Normale Supérieure (ENS) Rennes, the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche (MENESR) and the ARC Foundation for Cancer Research. AR was supported by the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche (MENESR). EH was supported by Oregon Agricultural Experimental Station Hatch Funds (OR00735).
Author information
Authors and Affiliations
Contributions
AR and EH designed research; SD, JG, CPW, AI, ES, and MCGC performed experiments; SD, CPW, ES, and MCGC analyzed data; SD and AR wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dufresne, S., Guéritat, J., Wong, C.P. et al. Exercise training as a modulator of epigenetic events in prostate tumors. Prostate Cancer Prostatic Dis 25, 119–122 (2022). https://doi.org/10.1038/s41391-021-00380-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41391-021-00380-x
This article is cited by
-
A review of exercise-induced epigenetic modifications in prostate tissue: implications for gene expression and tumor progression in prostate cancer
Cancer Cell International (2025)
-
Ovarian epigenetics modifications following lifestyle interventions by exercise and alternate-day feeding in letrozole-induced PCOS rats
Scientific Reports (2025)
-
Exercise and survival benefit in cancer patients: evidence from a comprehensive meta-analysis
GeroScience (2025)
-
The effects of exercise on epigenetic modifications: focus on DNA methylation, histone modifications and non-coding RNAs
Human Cell (2024)
-
Working hard or hardly working? A brief commentary of latest research on exercise and prostate cancer
Prostate Cancer and Prostatic Diseases (2023)