Abstract
Background
Little is known about the true rate of pathogenic (P)/likely pathogenic (LP) germline alterations in Hispanic men with prostate cancer as most studies analyzing the prevalence of P/LP germline alterations were performed in a largely non-Hispanic white population (NHW).
Methods
We performed a retrospective analysis of two separate cohorts of men with prostate cancer: (1) a multicenter cohort of 17,256 men who underwent germline testing in a CLIA-certified laboratory and (2) a single-center cohort of all men eligible for germline testing between 2018 and 2020. The proportions of P/LP alterations and variants of uncertain significance (VUS) were computed. Fisher’s exact test was used to compare germline alteration rates for significance. A multivariate logistic regression was performed adjusting for demographic and clinical factors to examine factors associated with germline testing.
Results
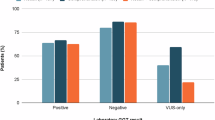
In the multicenter cohort, the rate of P/LP germline alterations among self-reported Hispanic men was 7.1%, which was lower than self-reported NHW men (9.7% vs. 7.1%, p = 0.058), but was not statistically significant. The VUS rate was significantly higher among the Hispanic cohort (21.5% vs. 16.6%, p = 0.005). In the single-center cohort, 136 Hispanic patients were eligible for testing of which 34 underwent germline testing (26.1%, N = 34/136). Of all prostate cancer patients in the single-center cohort undergoing germline testing (n = 173), the rate of P/LP alterations in Hispanic patients was not significantly different compared to NHW patients (14.7% vs. 12.2%, p = 0.77). The rate of VUS in Hispanic patients was significantly higher than that of NHW patients (20.6% vs. 7.2%, p = 0.047).
Conclusion
The P/LP germline alteration rate in our cohorts was similar between Hispanic and NHW men. The rate of VUS was significantly higher in Hispanic men, a consequence of undertesting in minority populations. These data support that Hispanic men with prostate cancer should be screened for germline testing similar to NHW men.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Datasets on which the conclusions of this paper relay are available to readers promptly upon request.
Change history
29 April 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41391-022-00539-0
References
Cancer.org. 2021. Key statistics for prostate cancer | prostate cancer facts. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed 27 Aug 2021.
Siegel DA, O’Neil ME, Richards TB, Dowling NF, Weir HK. Prostate cancer incidence and survival, by stage and race/ethnicity—United States, 2001–2017. MMWR Morb Mortal Wkly Rep. 2020;69:1473–80.
Dobbs RW, Malhotra NR, Abern MR, Moreira DM. Prostate cancer disparities in Hispanics by country of origin: a nationwide population-based analysis. Prostate Cancer Prostatic Dis. 2019;22:159–67.
Miller KD, Ortiz AP, Pinheiro PS, Bandi P, Minihan A, Fuchs HE, et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J Clin. 2021;71:466–87.
Brandão A, Paulo P, Teixeira MR. Hereditary predisposition to prostate cancer: from genetics to clinical implications. Int J Mol Sci. 2020;21:5036.
Mucci LA, Hjelmborg JB, Harris JR, Czene K, Havelick DJ, Scheike T, et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA. 2016;315:68–76.
Bratt O, Drevin L, Akre O, Garmo H, Stattin P. Family history and probability of prostate cancer, differentiated by risk category: a nationwide population-based study. J Natl Cancer Inst. 2016;108:djw110.
Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443–53.
[Guideline] Prostate Cancer. National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Version 3.2020. 2020. Accessed 9 Dec 2021.
Esplin ED, Cahn DJ, Mazzarella B, Pieczonka CM, Gazi M, Belkoff LH, et al. Underdiagnosis of germline genetic prostate cancer: are genetic testing guidelines an aid or an impediment?. J Clin Oncol. 2021;39 10504:15.
Nicolosi P, Ledet E, Yang S, Michalski S, Freschi B, O’Leary E, et al. Prevalence of germline variants in prostate cancer and implications for current genetic testing guidelines. JAMA Oncol. 2019;5:523–8.
Kwon DHK, Borno HT, Cheng HH, Zhou AY, Small EJ. Ethnic disparities among men with prostate cancer undergoing germline testing. Urol Oncol. 2020:80.e1–7.
Giri VN, Hegarty SE, Hyatt C, O’Leary E, Garcia J, Knudsen KE, et al. Germline genetic testing for inherited prostate cancer in practice: implications for genetic testing, precision therapy, and cascade testing. Prostate. 2019;79:333–9.
Kamath PN, Schlumbrecht MP, George S, Slomovitz BM, Koru-Sengul T, Miao F, et al. Identifying disparities in germline and somatic testing in patients with ovarian cancer in a University Health System. Gynecol Oncol. 2018;149:134.
Levy DE, Byfield SD, Comstock CB, Garber JE, Syngal S, Crown WH, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: black and Hispanic women particularly at risk. Genet Med. 2011;13:349–55.
Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93.
Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–102.
Abida W, Patnaik A, Campbell D, Shapiro J, Bryce AH, McDermott R, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020;38:3763–72.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13.
Samadder NJ, Riegert-Johnson D, Boardman L, Rhodes D, Wick M, Okuno S, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7:230–7.
Nykamp K, Anderson M, Powers M, Garcia J, Herrera B, Ho YY, et al. Sherloc: a comprehensive refinement of the ACMG-AMP variant classification criteria. Genet Med. 2017;19:1105–17.
Saulsberry K, Terry SF. The need to build trust: a perspective on disparities in genetic testing. Genet Test Mol Biomark. 2013;17:647–8.
Glenn B, Chawla N, Bastani R. Barriers to genetic testing for breast cancer risk among ethnic minority women: an exploratory study. Ethnicity Dis. 2012;22:267–73.
Hsiao SJ, Sirecy A, Pendrick D, Freeman C, Yang J, Schwartz GK. Clinical utility and reimbursement for expanded genomic panel testing in adult oncology. J Clin Oncol. 2019;37:6593.
Rana HQ, Stopfer JE, Petrucelli N, Koeller DR, Pirzadeh-Miller S, Reys B, et al. A randomized controlled trial of video-education or in-person genetic counseling for men with prostate cancer (ProGen). J Clin Oncol. 2020;38 15_1507.
Acknowledgements
We thank Invitae for providing data.
Author information
Authors and Affiliations
Contributions
RRM, JS, and EP contributed to the study concept and design. RRM, EP, and JS drafted the paper. All authors were involved in the acquisition, analysis, or interpretation of data, and participated in critical revision of the paper for important intellectual content and provided approval of the final paper. RRM supervised the study.
Corresponding author
Ethics declarations
Competing interests
RRM received research funding from Bayer, Pfizer, Tempus; serves on Advisory Board for AstraZeneca, Bayer, Bristol Myers Squibb, Calithera, Exelixis, Janssen, Merck, Novartis, Pfizer, Sanofi, Tempus; is a consultant for Dendreon, Myovant, Sorrento Therapeutics, Vividion; serves on the molecular tumor board at Caris. RLN is a consultant and stockholder in Maze Therapeutics and Genome Medical and a consultant for Pfizer Pharmaceuticals. SMN, KEH, EDE, and RLN are employees and stockholders of Invitae.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to add author Juan Javier-Desloges.
Rights and permissions
About this article
Cite this article
Pan, E., Shaya, J., Madlensky, L. et al. Germline alterations among Hispanic men with prostate cancer. Prostate Cancer Prostatic Dis 25, 561–567 (2022). https://doi.org/10.1038/s41391-022-00517-6
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41391-022-00517-6
This article is cited by
-
Disparities in prostate cancer diagnosis and management: recognizing that disparities exist at all junctures along the prostate cancer journey
Prostate Cancer and Prostatic Diseases (2023)
-
Performance of clinical risk scores and prediction models to identify pathogenic germline variants in patients with advanced prostate cancer
World Journal of Urology (2023)