Abstract
Background
The onset of castration-resistance is associated with dismal outcomes in patients with prostate cancer (PCa). Metastasis directed therapy has been investigated in multiple disease settings and may improve outcomes in selected patients. Our systematic review aims to summarize evidence with stereotactic body radiotherapy (SBRT) in castration-resistant prostate cancer (CRPC).
Methods
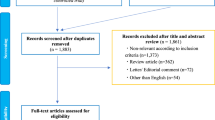
The literature search was performed on March 2024, on Pubmed, using the keywords “SBRT” AND “CRPC”, and “stereotactic ablative radiotherapy (SABR)” AND “CRPC”. This search retrieved a total of 108 articles, 19 were included.
Results
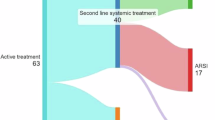
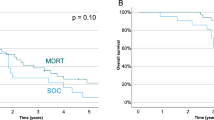
The literature is largely dominated by retrospective series. In men with metachronous oligoprogression, SBRT with androgen receptor pathway inhibitor significantly increased progression-free survival (PFS) including biochemical progression-free survival in a randomized phase II trial (hazard ratio of 0.35, p < 0.001). In patients continuing ADT, the bPFS ranged between 9.5 months to 17.9 months, and next systemic treatment-free survival (NEST-FS) reached up to 2 years. In men with induced oligoprogression, SBRT enabled NEST-FS up to 3 years. SBRT was well tolerated, with less than 5% grade 3 toxicity reported across studies.
Conclusion
In the population of patients with oligometastatic CRPC, SBRT enables long-term biochemical response and PFS. In the oligoprogressive setting, SBRT could be integrated to prolong the duration and efficacy of systemic therapies. Nevertheless, the level of evidence remains very low and inclusion within prospective trials remain the preferred option for this population of patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dutt SS, Gao AC. Molecular mechanisms of castration-resistant prostate cancer progression. Future Oncol. 2009;5:1403–13.
Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79:150–8.
Sternberg CN, Fizazi K, Saad F, Shore ND, De Giorgi U, Penson DF, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197–206.
Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383:1040–9.
Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PFA, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels J-P, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54.
Nuhn P, De Bono JS, Fizazi K, Freedland SJ, Grilli M, Kantoff PW, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2019;75:88–99.
Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18–28.
Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–66.
Hannan R, Christensen M, Hammers H, Christie A, Paulman B, Lin D, et al. Phase II trial of stereotactic ablative radiation for oligoprogressive metastatic kidney cancer. Eur Urol Oncol. 2022;5:216–24.
Cheung P, Patel S, North SA, Sahgal A, Chu W, Soliman H, et al. Stereotactic radiotherapy for oligoprogression in metastatic renal cell cancer patients receiving tyrosine kinase inhibitor therapy: a phase 2 prospective multicenter study. Eur Urol. 2021;80:693–700.
Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): five-year results of a randomized phase II trial. J Clin Oncol. 2020;38:10.
Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6:650.
Harrow S, Palma DA, Olson R, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic radiation for the comprehensive treatment of oligometastases (SABR-COMET)—extended long-term outcomes. Int J Radiat Oncol Biol Phys. 2022;114:611–6.
Francolini G, Allegra AG, Detti B, Di Cataldo V, Caini S, Bruni A, et al. Stereotactic body radiation therapy and abiraterone acetate for patients affected by oligometastatic castrate-resistant prostate cancer: a randomized phase II trial (ARTO). J Clin Oncol. 2023;41:5561–8.
Pan J, Wei Y, Zhang T, Liu C, Hu X, Zhao J, et al. Stereotactic radiotherapy for lesions detected via 68Ga-prostate-specific membrane antigen and 18F-fluorodexyglucose positron emission tomography/computed tomography in patients with nonmetastatic prostate cancer with early prostate-specific antigen progression on androgen deprivation therapy: a prospective single-center study. Eur Urol Oncol. 2022;5:420–7.
Henkenberens C, Derlin T, Bengel F, Ross TL, Kuczyk MA, Giordano FA, et al. Efficacy of PSMA PET-guided radiotherapy for oligometastatic castrate-resistant prostate cancer. Front Oncol. 2021;11:664225.
Onal C, Ozyigit G, Oymak E, Guler OC, Tilki B, Hurmuz P, et al. Stereotactic radiotherapy to oligoprogressive lesions detected with 68Ga-PSMA-PET/CT in castration-resistant prostate cancer patients. Eur J Nucl Med Mol Imaging. 2021;48:3683–92.
Lohaus F, Zöphel K, Löck S, Wirth M, Kotzerke J, Krause M, et al. Can local ablative radiotherapy revert castration-resistant prostate cancer to an earlier stage of disease? Eur Urol. 2019;75:548–51.
Berghen C, Joniau S, Ost P, Poels K, Everaerts W, Decaestecker K, et al. Progression-directed therapy for oligoprogression in castration-refractory prostate cancer. Eur Urol Oncol. 2021;4:305–9.
Zhang H, Orme JJ, Abraha F, Stish BJ, Lowe VJ, Lucien F, et al. Phase II evaluation of stereotactic ablative radiotherapy (SABR) and immunity in 11C-Choline-PET/CT–identified oligometastatic castration-resistant prostate Cancer. Clin Cancer Res. 2021;27:6376–83.
Chamois J, Septans AL, Schipman B, Gross E, Blanchard N, Passerat V, et al. Metastases-directed radiotherapy in castration resistant oligo metastatic prostate cancer: a multicentric retrospective study from the French group COLib. Clin Transl Radiat Oncol. 2024;46:100762.
Tsai CJ, Yang JT, Shaverdian N, Patel J, Shepherd AF, Eng J, et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): an open-label, randomised, controlled, phase 2 study. Lancet. 2024;403:171–82.
Greco C, Pares O, Pimentel N, Louro V, Morales J, Nunes B, et al. Phenotype-oriented ablation of oligometastatic cancer with single dose radiation therapy. Int J Radiat Oncol Biol Phys. 2019;104:593–603.
Rans K, Joniau S, Berghen C, Goffin K, Dumez H, Haustermans K, et al. Progression-directed therapy in oligoprogressive castration-resistant prostate cancer: final results from the prospective, single-arm, phase 2 MEDCARE trial. Eur Urol Oncol. 2024. https://doi.org/10.1016/j.euo.2024.04.003.
Pezzulla D, Macchia G, Cilla S, Buwenge M, Ferro M, Bonome P, et al. Stereotactic body radiotherapy to lymph nodes in oligoprogressive castration-resistant prostate cancer patients: a post hoc analysis from two phase I clinical trials. Clin Exp Metastasis. 2021;38:519–26.
Triggiani L, Mazzola R, Magrini SM, Ingrosso G, Borghetti P, Trippa F, et al. Metastasis-directed stereotactic radiotherapy for oligoprogressive castration-resistant prostate cancer: a multicenter study. World J Urol. 2019;37:2631–7.
La Vecchia M, Fazio I, Borsellino N, Lo Casto A, Galanti D. Stereotactic body radiotherapy in oligoprogressive metastatic castration-resistant prostate cancer during abiraterone or enzalutamide. Tumor J. 2023;109:413–7.
Ingrosso G, Detti B, Fodor A, Caini S, Borghesi S, Triggiani L, et al. Stereotactic ablative radiotherapy in castration-resistant prostate cancer patients with oligoprogression during androgen receptor-targeted therapy. Clin Transl Oncol. 2021;23:1577–84. https://doi.org/10.1007/s12094-021-02553-5.
Onal C, Kose F, Ozyigit G, Aksoy S, Oymak E, Muallaoglu S, et al. Stereotactic body radiotherapy for oligoprogressive lesions in metastatic castration-resistant prostate cancer patients during abiraterone/enzalutamide treatment. Prostate. 2021;81:543–52.
Baron D, Pasquier D, Pace-Loscos T, Vandendorpe B, Schiappa R, Ortholan C, et al. Stereotactic body radiation therapy to postpone systemic therapy escalation for castration-resistant prostate cancer: a multicenter retrospective analysis. Clin Transl Radiat Oncol. 2024;45:100710.
Franzese C, Perrino M, Marzo MA, Badalamenti M, Baldaccini D, D’Agostino G, et al. Oligoprogressive castration-resistant prostate cancer treated with metastases-directed stereotactic body radiation therapy: predictive factors for patients’ selection. Clin Exp Metastasis. 2022;39:449–57.
Deek MP, Taparra K, Phillips R, Velho PI, Gao RW, Deville C, et al. Metastasis-directed therapy prolongs efficacy of systemic therapy and improves clinical outcomes in oligoprogressive castration-resistant prostate cancer. Eur Urol Oncol. 2021;4:447–55.
de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–102.
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–103.
Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5:471–8.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366. https://doi.org/10.1056/NEJMoa1200690.
Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35:40–7.
Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJM, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–12.
Kwan EM, Spain L, Anton A, Gan CL, Garrett L, Chang D, et al. Avelumab combined with stereotactic ablative body radiotherapy in metastatic castration-resistant prostate cancer: the phase 2 ICE-PAC clinical trial. Eur Urol. 2022;81:253–62.
Fakhrejahani F, Madan RA, Dahut WL, Karzai F, Cordes LM, Schlom J, et al. Avelumab in metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2017;35:159.
Hannan R, Dohopolski MJ, Pop LM, Mannala S, Watumull L, Mathews D, et al. Phase II trial of sipuleucel-T and stereotactic ablative body radiation for patients with metastatic castrate-resistant prostate cancer. Biomedicines. 2022;10:1419.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22.
Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II—2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263–82.
Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–25.
James ND, Spears MR, Clarke NW, Dearnaley DP, De Bono JS, Gale J, et al. Survival with newly diagnosed metastatic prostate cancer in the ‘Docetaxel Era’: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur Urol. 2015;67:1028–38.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18.
D’Angelillo RM, Francolini G, Ingrosso G, Ravo V, Triggiani L, Magli A, et al. Consensus statements on ablative radiotherapy for oligometastatic prostate cancer: a position paper of Italian Association of Radiotherapy and Clinical Oncology (AIRO). Crit Rev Oncol Hematol. 2019;138:24–8.
Zilli T, Achard V, Dal Pra A, Schmidt-Hegemann N, Jereczek-Fossa BA, Lancia A, et al. Recommendations for radiation therapy in oligometastatic prostate cancer: an ESTRO-ACROP Delphi consensus. Radiother Oncol. 2022;176:199–207.
Roach PJ, Francis R, Emmett L, Hsiao E, Kneebone A, Hruby G, et al. The impact of 68 Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian Prospective Multicenter Study. J Nucl Med. 2018;59:82–8.
Fendler WP, Weber M, Iravani A, Hofman MS, Calais J, Czernin J, et al. Prostate-specific membrane antigen ligand positron emission tomography in men with nonmetastatic castration-resistant prostate cancer. Clin Cancer Res. 2019;25:7448–54.
Chen W, Li L, Ji S, Song X, Lu W, Zhou T. Evaluation of potential surrogate endpoints for prediction of overall survival in patients with castration-resistant prostate cancer: trial-level meta-analysis. Eur J Clin Pharm. 2019;75:1521–32.
Author information
Authors and Affiliations
Contributions
Jennifer Le Guevelou: conceived and designed the analysis, collected the data, contributed in data analysis, contributed in the writing process; Francesco Cuccia: conceived and designed the analysis, collected the data, contributed in data analysis, contributed in the writing process; Ronan Flippot: contributed in data analysis, contributed in the writing process; Giuseppe Ferrera: contributed in data analysis, contributed in the writing process; Mario Terlizzi: contributed in data analysis, contributed in the writing process; Thomas Zilli: contributed in data analysis, contributed in the writing process; Renaud De Crevoisier: contributed in data analysis, contributed in the writing process; Jean-Michel Hannoun-Levi: contributed in data analysis, contributed in the writing process; Stephane Supiot: contributed in data analysis, contributed in the writing process; Paul Sargos: contributed in data analysis, contributed in the writing process; David Pasquier: conceived and designed the analysis, contributed in data analysis, contributed in the writing process.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le Guevelou, J., Cuccia, F., Flippot, R. et al. The current landscape of stereotactic body radiation therapy for metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 28, 546–556 (2025). https://doi.org/10.1038/s41391-024-00862-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41391-024-00862-8
This article is cited by
-
The changing face of castrate resistant prostate cancer
Prostate Cancer and Prostatic Diseases (2024)
-
MDT perspective: innovative applications of stereotactic body radiation therapy in metastatic castration-resistant prostate cancer
Prostate Cancer and Prostatic Diseases (2024)