Abstract
Purpose
To investigate the detection and predictors of prostate cancer (PCA) and clinically significant prostate cancer (csPCA) in patients with positive multiparametric MRI (mpMRI) followed by a negative MRI – guided target biopsy (TB) and systematic biopsy (SB).
Materials and methods
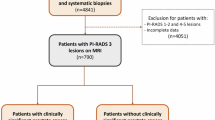
This retrospective multicenter study included 694 patients from 10 tertiary referral centers with an initial positive mpMRI (PI-RADS ≥ 3) and negative results on both MRI-TB and SB. Patients were classified into three groups based on follow-up: Group 1 (prostate re-biopsy without new mpMRI), Group 2 (standardized second prostate mpMRI and subsequent re-biopsy), and Group 3 (follow-up with mpMRIs and biopsy based on clinical and radiological triggers). The primary outcomes were the detection of any PCA and csPCA during follow up. Study groups were compared according to their probability of PCA and csPCA assessed with the ERSPC-MRI risk calculator. Statistical analysis included Kaplan – Meier analysis, Cox regression, and multivariable analysis for the detection of (cs)PCa.
Results
The overall detection of PCA and csPCA was 26.8% and 19.3%, respectively, with varying rates in different PI-RADS groups. Group 3 had the highest 2-year and 5-year PCA–free survival (94 and 84%) and csPCA – free survival (96 and 86%). Multivariable analysis revealed a significantly higher risk of PCA and csPCA in Group 1 and 2 compared to Group 3 (p < 0.01). Clinical and radiological predictors for PCA and csPCA included higher age, prostate volume, PI-RADS score, the presence of atypical small acinar proliferation (ASAP), and a smaller number of TB and SB performed during the initial biopsy. Study limitations, include the retrospective design and reliance on clinical and radiological triggers for follow–up decisions.
Conclusions
Patients with positive mpMRI but negative TB and SB results exhibit varying rates of PCA and csPCA depending on the follow up scheme. Tailored follow-up strategies are essential for optimal management in this clinical scenario.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available for sharing but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of authors.
References
Vittori G, Bacchiani M, Grosso AA, Raspollini MR, Giovannozzi N, Righi L, et al. Computer-aided diagnosis in prostate cancer: a retrospective evaluation of the Watson Elementary® system for preoperative tumor characterization in patients treated with robot-assisted radical prostatectomy. World J Urol. 2023;41:435–41.
Checcucci E, Rosati S, De Cillis S, Vagni M, Giordano N, Piana A, et al. Artificial intelligence for target prostate biopsy outcomes prediction the potential application of fuzzy logic. Prostate Cancer Prostatic Dis. 2022;25:359–62.
Denijs FB, van Harten MJ, Meenderink JJL, Leenen RCA, Remmers S, Venderbos LDF et al. Risk calculators for the detection of prostate cancer: a systematic review. Prostate Cancer Prostatic Dis 2024; 27. https://doi.org/10.1038/S41391-024-00852-W.
Li E V., Busza AM, Siddiqui MR, Aguiar JA, Keeter MK, Neill C et al. Detection of clinically significant prostate cancer following initial omission of biopsy in multiparametric MRI era. Prostate Cancer Prostatic Dis 2024. https://doi.org/10.1038/S41391-024-00853-9.
Kasivisvanathan V, Stabile A, Neves JB, Giganti F, Valerio M, Shanmugabavan Y, et al. Magnetic resonance imaging-targeted biopsy versus systematic biopsy in the detection of prostate cancer: a systematic review and meta-analysis. Eur Urol. 2019;76:284–303.
Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Brunckhorst O, Darraugh J, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer—2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2024;86:148–63.
Wysock JS, Mendhiratta N, Zattoni F, Meng X, Bjurlin M, Huang WC, et al. Predictive value of negative 3T multiparametric magnetic resonance imaging of the prostate on 12-core biopsy results. BJU Int. 2016;118:515–20.
Sathianathen NJ, Omer A, Harriss E, Davies L, Kasivisvanathan V, Punwani S, et al. Negative predictive value of multiparametric magnetic resonance imaging in the detection of clinically significant prostate cancer in the prostate imaging reporting and data system era: a systematic review and meta-analysis. Eur Urol. 2020;78:402–14.
Pepe P, Garufi A, Priolo GD, Pennisi M, Fraggetta F. Early second round targeted biopsy of PI-RADS score 3 or 4 in 256 men with persistent suspicion of prostate cancer. Vivo. 2019;33:897–901.
Barletta F, Stabile A, Mazzone E, Brembilla G, Sorce G, Pellegrino F, et al. How to optimize follow-up in patients with a suspicious multiparametric MRI and a subsequent negative targeted prostate biopsy. Results from a large, single-institution series. Urol Oncol. 2022;40:103.e17–103.e24.
Meng X, Chao B, Chen F, Huang R, Taneja SS, Deng FM. Followup of Men with PI-RADSTM 4 or 5 abnormality on prostate magnetic resonance imaging and nonmalignant pathological findings on initial targeted prostate biopsy. J Urol. 2021;205:748–54.
Wallström J, Geterud K, Kohestani K, Maier SE, Pihl CG, Socratous A, et al. Prostate cancer screening with magnetic resonance imaging: results from the second round of the Göteborg prostate cancer screening 2 trial. Eur Urol Oncol. 2022;5:54–60.
Costa DN, Kay FU, Pedrosa I, Kolski L, Lotan Y, Roehrborn CG, et al. An initial negative round of targeted biopsies in men with highly suspicious multiparametric magnetic resonance findings does not exclude clinically significant prostate cancer—Preliminary experience. Urol Onco. 2017;35:149.e15–149.e21.
Venderink W, Jenniskens SFM, Michiel Sedelaar JP, Tamada T, Fütterer JJ. Yield of repeat targeted direct in-bore magnetic resonance-guided prostate biopsy (MRGB) of the same lesions in men having a prior negative targeted MRGB. Korean J Radio. 2018;19:733–41.
Grivas N, Lardas M, Espinós EL, Lam TB, Rouviere O, Mottet N, et al. Prostate cancer detection percentages of repeat biopsy in patients with positive multiparametric magnetic resonance imaging (prostate imaging reporting and data system/likert 3–5) and negative initial biopsy. a mini systematic review. Eur Urol. 2022;82:452–7.
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging - Reporting And Data System: 2015, Version 2. Eur Urol. 2016;69:16–40.
de Rooij M, Israël B, Tummers M, Ahmed HU, Barrett T, Giganti F, et al. ESUR/ESUI consensus statements on multi-parametric MRI for the detection of clinically significant prostate cancer: quality requirements for image acquisition, interpretation and radiologists’ training. Eur Radio. 2020;30:5404–16.
Alberts AR, Roobol MJ, Verbeek JFM, Schoots IG, Chiu PK, Osses DF, et al. Prediction of high-grade prostate cancer following multiparametric magnetic resonance imaging: improving the Rotterdam European randomized study of screening for prostate cancer risk calculators. Eur Urol. 2019;75:310–8.
Emmett L, Buteau J, Papa N, Moon D, Thompson J, Roberts MJ, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): a prospective multicentre study. Eur Urol. 2021;80:682–9.
Meissner VH, Rauscher I, Schwamborn K, Neumann J, Miller G, Weber W, et al. Radical prostatectomy without prior biopsy following multiparametric magnetic resonance imaging and prostate-specific membrane antigen positron emission tomography. Eur Urol. 2022;82:156–60.
Tang W, Tang Y, Qi L, Zhang Y, Tang G, Gao X, et al. Benign prostatic hyperplasia-related false-positive of prostate-specific membrane antigen-positron emission tomography in the diagnosis of prostate cancer: the Achilles’ heel of biopsy-free radical prostatectomy? J Urol. 2023;210:845–55.
Zattoni F, Pereira LJP, Marra G, Valerio M, Olivier J, Puche-Sanz I, et al. The impact of a second MRI and re-biopsy in patients with initial negative mpMRI-targeted and systematic biopsy for PIRADS ≥ 3 lesions. World J Urol. 2023;41:3357–66.
Gordetsky JB, Ullman D, Schultz L, Porter KK, del Carmen Rodriguez Pena M, Calderone CE, et al. Histologic findings associated with false-positive multiparametric magnetic resonance imaging performed for prostate cancer detection. Hum Pathol. 2019;83:159–65.
Hupe MC, Offermann A, Tharun L, Fürschke A, Frydrychowicz A, Garstka N, et al. Histomorphological analysis of false positive PI-RADS 4 and 5 lesions. Urol Oncol. 2020;38:636.e7–636.e12.
Zattoni F, Rajwa P, Miszczyk M, Fazekas T, Carletti F, Carrozza S et al. Transperineal versus transrectal magnetic resonance imaging-targeted prostate biopsy: a systematic review and meta-analysis of prospective studies. Eur Urol Oncol 2024. https://doi.org/10.1016/J.EUO.2024.07.009.
Zattoni F, Marra G, Kasivisvanathan V, Grummet J, Nandurkar R, Ploussard G, et al. The Detection of Prostate Cancer with Magnetic Resonance Imaging-Targeted Prostate Biopsies is Superior with the Transperineal vs. the Transrectal Approach. A European Association of Urology-Young Academic Urologists Prostate Cancer Working Group Multi-Institutional Study. J Urol. 2022;208:830–7.
Zattoni F, Marra G, Martini A, Kasivisvanathan V, Grummet J, Harkin T et al. Is There an Impact of Transperineal Versus Transrectal Magnetic Resonance Imaging-targeted Biopsy on the Risk of Upgrading in Final Pathology in Prostate Cancer Patients Undergoing Radical Prostatectomy? An European Association of Urology-Young Academic Urologists Prostate Cancer Working Group Multi-institutional Study. Eur Urol Focus 2023. https://doi.org/10.1016/J.EUF.2023.01.016.
Author information
Authors and Affiliations
Contributions
Protocol/project development: F Zattoni, G Gandaglia, G Novara, R C N van den Bergh, A Briganti. Data collection or management: LJ.Paulino Pereira, G Marra, M Valerio, J Olivier, I Puche – SanzI, M Maggi, R Campi, D Amparore, S De Cillis, Z Junlong, H Guo, G La Bombarda, A Fuschi., A Veccia, F Ditonno, A Marquis, F Barletta, R Leni, S Serni, Data analysis: F Zattoni, G Novara, F Dal Moro, Sebastiaan Remmers, Monique J. Roobol. Manuscript writing/editing: F Zattoni, G Novara, R C N van den Bergh, A Antonelli, K Veeru, P Rajwa
Corresponding author
Ethics declarations
Competing interests
The authors declared that they have no conflict of interests. All included patients undergoing radical treatment provided written informed consent for surgery. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional review board apply to each center due to observational and retrospective nature of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zattoni, F., Gandaglia, G., van den Bergh, R.C.N. et al. Follow-up on patients with initial negative mpMRI target and systematic biopsy for PI-RADS ≥ 3 lesions – an EAU-YAU study enhancing prostate cancer detection. Prostate Cancer Prostatic Dis 28, 435–443 (2025). https://doi.org/10.1038/s41391-024-00904-1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41391-024-00904-1
This article is cited by
-
Less is more: the “Safety Net Approach” balances precision and safety
Prostate Cancer and Prostatic Diseases (2025)
-
Follow-up on patients with initial negative mpMRI target and systematic biopsy for PI-RADS ≥ 3 lesions—an EAU-YAU study enhancing prostate cancer detection
Prostate Cancer and Prostatic Diseases (2025)