Abstract
Objective
We sought to determine, in a prospective long term cohort, the prognostic value of negative MR imaging with respect to upgrading and need for intervention in men on AS.
Method
A long term prospective single centre study of men on Active surveillance with MR imaging. Primary outcome was upgrading on biopsy and rate of intervention. After incorporation of MRI into the AS protocol in 2013, men with negative imaging underwent systematic biopsy only for cause.
Results
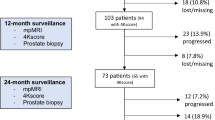
Five hundred and thirty AS patients had one or more MRI, with median follow-up of 8.5 years. At baseline, 39 patients (7.4%) had negative MRIs 161 (30%) equivocal, and 330 (62%) had a positive MRI. Two hundred and twenty-nine patients were upgraded; 5% with invisible lesions, 34% with PiRADS 3, and 52% with PiRADS 4–5. Two hundred and twenty-nine (43%) of the 530 men were eventually treated. No patient with a negative PiRADS was treated, vs 36% with PiRADS 3 and 52% with PiRADS 4–5 (p < 0.001). In 331 men with serial MRIs, upgrading occurred in 46% of men with stable or improved MRI, and 57% in those with MRI progression. In the 70 patients whose MRI improved from PiRADS 4–5 to 3, 46% were upgraded. No patients who transitioned from PiRADS 3–5 to 1–2 were upgraded. Time to grade progression was highly inversely correlated with PIRADS score.
Conclusion
MRI invisible cancers demonstrated dramatically reduced rates of progression and no patient required intervention. Despite the absence of routine biopsies in the MR negative group, none of these patients progressed over time to GG ≥ 3 or metastatic disease. This suggests that, in men on active surveillance, image guided management, restricting biopsies to targeted biopsies of regions of interest, is sufficient to identify clinically significant cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schaeffer EM, Srinivas S, Adra N, An Y, Barocas D, Bitting R, et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:1067–1096.
Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7.
Tosoian JJ, Mamawala M, Epstein JI, Landis P, Macura KJ, Simopoulos DN. et al. Active Surveillance of Grade Group 1 Prostate Cancer: Long-term Outcomes from a Large Prospective Cohort. Eur Urol. 2020;77:675–82. https://doi.org/10.1016/j.eururo.2019.12.017.
Courtney PT, Deka R, Kotha NV, Cherry DR, Salans MA, Nelson TJ, et al. Metastasis and Mortality in Men With Low- and Intermediate-Risk Prostate Cancer on Active Surveillance. J Natl Compr Canc Netw. 2022;20:151–9.
Lehto TK, Pylväläinen J, Sandeman K, Kenttämies A, Nordling S, Mills IG, et al. Histomic and transcriptomic features of MRI-visible and invisible clinically significant prostate cancers are associated with prognosis. Int J Cancer. 2024;154:926–39.
Wibmer AG, Lefkowitz RA, Lakhman Y, Chaim J, Nikolovski I, Sala E, et al. MRI-Detectability of Clinically Significant Prostate Cancer Relates to Oncologic Outcomes After Prostatectomy. Clin Genitourin Cancer. 2022;20:319–25.
Pachynski RK, Kim EH, Miheecheva N, Kotlov N, Ramachandran A, Postovalova E, et al. Single-cell Spatial Proteomic Revelations on the Multiparametric MRI Heterogeneity of Clinically Significant Prostate Cancer. Clin Cancer Res. 2021;27:3478–90.
Ankerst DP, Xia J, Thompson IM Jr, Hoefler J, Newcomb LF, Brooks JD, et al. Precision Medicine in Active Surveillance for Prostate Cancer: Development of the Canary-Early Detection Research Network Active Surveillance Biopsy Risk Calculator. Eur Urol. 2015;68:1083–8.
Jiang Y, Meyers TJ, Emeka AA, Cooley LF, Cooper PR, Lancki N, et al. Genetic Factors Associated with Prostate Cancer Conversion from Active Surveillance to Treatment. HGG Adv. 2022;3:100070.
Li P, You S, Nguyen C, Wang Y, Kim J, Sirohi D, et al. Genes involved in prostate cancer progression determine MRI visibility. Theranostics. 2018;8:1752–65.
Houlahan KE, Salmasi A, Sadun TY, Pooli A, Felker ER, Livingstone J, et al. Molecular Hallmarks of Multiparametric Magnetic Resonance Imaging Visibility in Prostate Cancer. Eur Urol. 2019;76:18–23.
Olivier J, Li W, Nieboer D, Helleman J, Roobol M, Gnanapragasam V, et al. Prostate Cancer Patients Under Active Surveillance with a Suspicious Magnetic Resonance Imaging Finding Are at Increased Risk of Needing Treatment: Results of the Movember Foundation’s Global Action Plan Prostate Cancer Active Surveillance (GAP3) Consortium. Eur Urol Open Sci. 2022;35:59–67.
Luiting HB, Remmers S, Boevé ER, Valdagni R, Chiu PK, Semjonow A, et al. A Multivariable Approach Using Magnetic Resonance Imaging to Avoid a Protocol-based Prostate Biopsy in Men on Active Surveillance for Prostate Cancer-Data from the International Multicenter Prospective PRIAS Study. Eur Urol Oncol. 2022;5:651–8.
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021;79:243–62.
Klotz L, Chin C, Black P, Finelli A, Anidjar M, Haider M. Magnetic Resonance Imaging–Targeted Versus Systematic Prostate Biopsies: 2-year Follow-up of a Prospective Randomized Trial (PRECISE). Eur Urol Oncol. 2024;7:456–61.
Drost FH, Osses D, Nieboer D, Bangma CH, Steyerberg EW, Roobol MJ, et al. Prostate Magnetic Resonance Imaging, with or Without Magnetic Resonance Imaging-targeted Biopsy, and Systematic Biopsy for Detecting Prostate Cancer: A Cochrane Systematic Review and Meta-analysis. Eur Urol. 2020;77:78–94.
Avolio PP, Lughezzani G, Paciotti M, Maffei D, Uleri A, Frego N, et al. The use of 29 MHz transrectal micro-ultrasound to stratify the prostate cancer risk in patients with PI-RADS III lesions at multiparametric MRI: A single institutional analysis. Urol Oncol. 2021;39:832.e1–832.e7.
Stolk TT, de Jong IJ, Kwee TC, Luiting HB, Mahesh SVK, Doornweerd BHJ, et al. False positives in PIRADS (V2) 3, 4, and 5 lesions: relationship with reader experience and zonal location. Abdom Radio. 2019;44:1044–51.
Pickersgill NA, Vetter JM, Andriole GL, Shetty AS, Fowler KJ, Mintz AJ, et al. Accuracy and Variability of Prostate Multiparametric Magnetic Resonance Imaging Interpretation Using the Prostate Imaging Reporting and Data System: A Blinded Comparison of Radiologists. Eur Urol Focus. 2020;6:267–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Klotz, L., Loblaw, A., Zhang, L. et al. Prognostic value of MR visibility/invisibility in men on Active Surveillance. Prostate Cancer Prostatic Dis (2025). https://doi.org/10.1038/s41391-024-00932-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00932-x