Abstract
Background
Zoledronic acid (ZA) in combination with androgen deprivation therapy (ADT) has never proved additional activity in patients with advanced prostate cancer. However, conventional imaging is poorly reliable in monitoring disease response of metastatic bone lesions.
Methods
BonEnza is a randomized phase II multicenter clinical trial designed to compare activity of ADT plus Enzalutamide (E) plus/minus ZA in term of bone response rate by Whole-Body Diffusion-Weighted Magnetic Resonance Imaging (WB-DW-MRI). From February 2018 to June 2021, 126 patients with metastatic hormone-sensitive prostate cancer (mHSPC) and bone metastasis at bone scan were enrolled. Patients were randomized in a 1:1 to receive E 160 mg OD orally alone (E arm) or in combination with ZA 4 mg intravenously every 4 weeks (EZ arm). Primary endpoint of the study was overall response rate (ORR) in bone metastases, secondary endpoints were ORR with conventional imaging, progression free survival (PFS) and overall survival (OS). A logistic model was used to evaluate the association between treatment arm and ORR.
Results
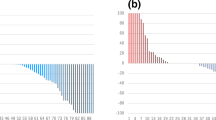
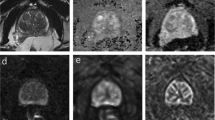
After a median follow-up of 31.9 months, according to an intent to treat analysis, the ORR was superimposable in both arms: 69.8% (95% Confidence Interval [CI]: 57.5–79.9%), Odds Ratio: 1.00 (95%CI 0.47–2.15; p > 0.9). No advantage in favor of EZ arm over E arm emerged either in terms of PFS (Hazard Ratio [HR] 0.77, 95%CI 0.44–1.37; p = 0.4) or OS (HR 1.09; 95%CI 0.54–2.2; p = 0.8). A main limitation of this study was the inability of WB-DW-MRI to evaluate disease response in 17 patients.
Conclusions
ZA did not improve bone response rate to E plus ADT in mHSPC patients. WB-DW-MRI is a reliable technique to evaluate the response of prostate cancer bone metastases to systemic therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Maiorano BA, De Giorgi U, Roviello G, Messina C, Altavilla A, Cattrini C, et al. Addition of androgen receptor-targeted agents to androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis. ESMO Open. 2022;7:100575. https://doi.org/10.1016/j.esmoop.2022.100575.
Armstrong AJ, Azad AA, Iguchi T, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40:1616–22. https://doi.org/10.1200/JCO.22.00193.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–31. https://doi.org/10.1056/NEJMoa1903835.
Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar RN, et al. NCCN task force report: bone health in cancer care. J Natl Compr Canc Netw. 2009;7:S1–32. https://doi.org/10.6004/jnccn.2009.0076.
Saylor PJ, Lee RJ, Smith MR. Emerging therapies to prevent skeletal morbidity in men with prostate cancer. J Clin Oncol. 2011;29:3705–14. https://doi.org/10.1200/JCO.2010.34.4994.
Wang L, Fang D, Xu J, Luo R. Various pathways of zoledronic acid against osteoclasts and bone cancer metastasis: a brief review. BMC Cancer. 2020;20:1059. https://doi.org/10.1186/s12885-020-07568-9.
Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–68. https://doi.org/10.1093/jnci/94.19.1458.
Smith MR, Halabi S, Ryan CJ, Hussain A, Vogelzang N, Stadler W, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32:1143–50. https://doi.org/10.1200/JCO.2013.51.6500.
Jones C, Dutey-Magni P, Murphy LR, Murray M, Brown JE, McCloskey E, et al. Incidence of fracture related hospitalisations in men with de novo high risk localised and metastatic hormone sensitive prostate cancer: Analysis of routinely collected healthcare data from the STAMPEDE docetaxel and zoledronic acid comparisons. Ann Oncol. 2023;34:S954–1000. https://doi.org/10.1016/annonc/annonc1334.
Cirak Y, Varol U, Atmaca H, Kisim A, Sezgin C, Karabulut B, et al. Zoledronic acid in combination with serine/threonine phosphatase inhibitors induces enhanced cytotoxicity and apoptosis in hormone-refractory prostate cancer cell lines by decreasing the activities of PP1 and PP2A. BJU Int. 2012;110:E1147–54. https://doi.org/10.1111/j.1464-410X.2012.11392.x.
Varol U, Degirmenci M, Karaca B, Atmaca H, Kisim A, Uzunoglu S, et al. Zoledronic acid increases cytotoxicity by inducing apoptosis in hormone and docetaxel-resistant prostate cancer cell lines. Tumour Biol. 2015;36:779–86. https://doi.org/10.1007/s13277-014-2682-6.
Gokalp C. Cytotoxic and anti-angiogenic effects of zoledronic acid in DU-145 and PC-3 prostate cancer cell lines. Mol Biol Rep. 2020;47:7675–83. https://doi.org/10.1007/s11033-020-05840-6.
Saad F, Shore N, Van Poppel H, Rathkopf DE, Smith MR, de Bono JS, et al. Impact of bone-targeted therapies in chemotherapy-naïve metastatic castration-resistant prostate cancer patients treated with abiraterone acetate: post hoc analysis of study COU-AA-302. Eur Urol. 2015;68:570–7. https://doi.org/10.1016/j.eururo.2015.04.032.
Mosavi F, Johansson S, Sandberg DT, Turesson I, Sörensen J, Ahlström H. Whole-body diffusion-weighted MRI compared with (18)F-NaF PET/CT for detection of bone metastases in patients with high-risk prostate carcinoma. AJR Am J Roentgenol. 2012;199:1114–20. https://doi.org/10.2214/AJR.11.8351.
Padhani AR, Makris A, Gall P, Collins DJ, Tunariu N, de Bono JS. Therapy monitoring of skeletal metastases with whole-body diffusion MRI. J Magn Reson Imaging. 2014;39:1049–78. https://doi.org/10.1002/jmri.24548.
Padhani AR, Lecouvet FE, Tunariu N, Koh DM, De Keyzer F, Collins DJ, et al. METastasis reporting and data system for prostate cancer: practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate cancer. Eur Urol. 2017;71:81–92. https://doi.org/10.1016/j.eururo.2016.05.033.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Prostate cancer clinical trials working group 3. trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–18. https://doi.org/10.1200/JCO.2015.64.2702.
Tombal B, Borre M, Rathenborg P, Werbrouck P, Van Poppel H, Heidenreich A, et al. Long-term efficacy and safety of enzalutamide monotherapy in hormone-naïve prostate cancer: 1- and 2-year open-label follow-up results. Eur Urol. 2015;68:787–94. https://doi.org/10.1016/j.eururo.2015.01.027.
Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3:35–44. https://doi.org/10.1002/sim.4780030106.
Vale CL, Burdett S, Rydzewska LHM, Albiges L, Clarke NW, Fisher D, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17:243–56. https://doi.org/10.1016/S1470-2045(15)00489-1. FebEpub 2015 Dec 21. Erratum in: Lancet Oncol. 2016 Feb;17(2):e46. 10.1016/S1470-2045(16)00020-6.
Oprea-Lager DE, Cysouw MCF, Boellaard R, Deroose CM, de Geus-Oei LF, Lopci E, et al. Bone metastases are measurable: the role of whole-body MRI and positron emission tomography. Front Oncol. 2021;11:772530. https://doi.org/10.3389/fonc.2021.772530.
Garcia-Ruiz A, Macarro C, Zacchi F, Morales-Barrera R, Grussu F, Casanova-Salas I, et al. Whole-body magnetic resonance imaging as a treatment response biomarker in castration-resistant prostate cancer with bone metastases: the iPROMET clinical trial. Eur Urol. 2024;86:272–4. https://doi.org/10.1016/j.eururo.2024.02.016.
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–86. https://doi.org/10.1200/JCO.19.00799.
Dalla Volta A, Valcamonico F, Zivi A, Procopio G, Sepe P, Del Conte G, et al. Whole-body diffusion-weighted magnetic resonance imaging for assessment of the bone response rate in patients with metastatic hormone-sensitive prostate cancer receiving enzalutamide. Eur Urol. 2024;86:268–71. https://doi.org/10.1016/j.eururo.2024.05.004.
Acknowledgements
FZ is supported by an AIRC fellowship Italy Post-Doc ID 29854-2023.
Funding
This research was partially funded by an investigator sponsored research grant from Astellas (grant number IT-72-RG-33/ISR004983). The funder had no role in design and conduct of the study, collection, management, analysis, and interpretation of the data and in preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
ADV: Investigation, Resources, Data Curation, Validation, Writing—Original Draft, Visualization, Project Administration. FV: Investigation, Resources, Writing—Review & Editing, Project Administration. AZ: Investigation, Resources, Writing—Review & Editing. GP: Investigation, Resources, Writing—Review & Editing. PS: Investigation, Writing—Review & Editing. GDC: Investigation, Resources, Writing—Review & Editing. NDM: Resources, Writing—Review & Editing. SF: Investigation, Writing—Review & Editing. SZ: Resources, Writing—Review & Editing. CM: Investigation, Resources, Writing—Review & Editing. EL: Investigation, Resources, Writing—Review & Editing. AR: Investigation, Resources, Writing—Review & Editing. MR: Resources, Writing—Review & Editing. SC: Formal analysis, Methodology, Resources, Validation, Writing—Review & Editing. FZ: Investigation, Writing—Review & Editing. GC: Software, Methodology, Writing—Review & Editing. NS: Resources, Writing—Review & Editing. RM: Conceptualization, Resources, Writing—Review & Editing. DF: Resources, Writing—Review & Editing. AB: Conceptualization, Resources, Validation, Writing—Review & Editing, Supervision, Project Administration, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dalla Volta, A., Valcamonico, F., Zivi, A. et al. Addition of zoledronic acid to enzalutamide and androgen deprivation therapy in metastatic hormone-sensitive prostate cancer: the randomized phase II BONENZA trial. Prostate Cancer Prostatic Dis (2025). https://doi.org/10.1038/s41391-025-00975-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-025-00975-8