Abstract
Background
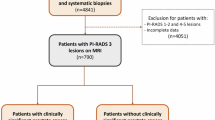
The use of prostate-specific antigen density (PSAd) in combination with multiparametric magnetic resonance imaging (mpMRI) of the prostate can improve accuracy of the prostate cancer (PCa) diagnostic pathway. However, it is not clear whether the performance characteristics of PSAd vary according to the index lesion location (ILL) on mpMRI.
Methods
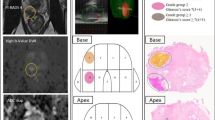
Overall, 2140 patients with positive mpMRI (prostate imaging reporting and data system [PI-RADS] ≥ 3) underwent mpMRI-targeted biopsy (TBx) plus systematic biopsy (SBx) at three tertiary referral centers. Multivariable logistic regression analysis (MVA) tested the interaction between PSAd and ILL (peripheral zone [PZ] vs transitional zone [TZ]) in predicting clinically significant PCa (csPCa, defined as ISUP grade group ≥2) at TBx. Non-parametric locally weighted scatterplots smoothing approach (LOWESS) explored the relationship between PSAd and csPCa according to ILL and stratifying by PI-RADS score.
Results
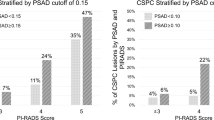
Median PSA was 6.7 ng/ml. ILL was PZ and TZ in 77% and 23% patients, respectively. Overall, 39% of csPCa cases were diagnosed at TBx. The association between PSAd and csPCa varied according to ILL (interaction test: p < 0.01). In patients with PI-RADS 3 lesions, csPCa incidence was <10% in cases of PSAd values < 0.05 ng/ml/ml and <0.13 ng/ml/ml for PZ and TZ lesions, respectively. Differently, in patients with PI-RADS ≥ 4, csPCa incidence was ≥20% regardless of PSAd value and ILL.
Conclusions
The likelihood of detecting csPCa in patients with PI-RADS 3 lesions is influenced by the combination of PSAd and ILL. Specifically, patients with PZ and TZ PI-RADS 3 lesions have an increased risk of csPCa for PSAd values ≥ 0.05 ng/ml/ml and ≥0.13 ng/ml/ml, respectively. Conversely, patients with PI-RADS ≥ 4 lesions have a non-negligible risk of csPCa regardless of PSAd and ILL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–77.
Ahdoot M, Wilbur AR, Reese SE, Lebastchi AH, Mehralivand S, Gomella PT, et al. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med. 2020;382:917–28.
Stabile A, Giganti F, Rosenkrantz AB, Taneja SS, Villeirs G, Gill IS, et al. Multiparametric MRI for prostate cancer diagnosis: current status and future directions. Nat Rev Urol. 2020;17:41–61.
Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Brunckhorst O, Darraugh J, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2024;86:148–63.
Pellegrino F, Stabile A, Sorce G, Mazzone E, Cannoletta D, Cirulli GO, et al. Variability of mpMRI diagnostic performance according to the upfront individual patient risk of having clinically significant prostate cancer. Prostate. 2024;84:473–8.
Mazzone E, Stabile A, Pellegrino F, Basile G, Cignoli D, Cirulli GO, et al. Positive predictive value of prostate imaging reporting and data system version 2 for the detection of clinically significant prostate cancer: a systematic review and meta-analysis. Eur Urol Oncol. 2021;4:697–713.
Pellegrino F, Tin AL, Martini A, Vertosick EA, Porwal SP, Stabile A, et al. Prostate-specific antigen density cutoff of 0.15 ng/ml/cc to propose prostate biopsies to patients with negative magnetic resonance imaging: efficient threshold or legacy of the past?. Eur Urol Focus. 2023;9:291–7.
Benson MC, Whang S, Pantuck A, Ring K, Kaplan SA, Olsson CA, et al. Prostate specific antigen density: a means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol. 1992;147:815–6.
Distler FA, Radtke JP, Bonekamp D, Kesch C, Schlemmer HP, Wieczorek K, et al. The value of PSA density in combination with PI-RADSTM for the accuracy of prostate cancer prediction. J Urol. 2017;198:575–82.
Oishi M, Shin T, Ohe C, Nassiri N, Palmer SL, Aron M, et al. Which patients with negative magnetic resonance imaging can safely avoid biopsy for prostate cancer?. J Urol. 2019;201:268–76.
Schoots IG, Padhani AR. Risk-adapted biopsy decision based on prostate magnetic resonance imaging and prostate-specific antigen density for enhanced biopsy avoidance in first prostate cancer diagnostic evaluation. BJU Int. 2021;127:175–8.
Pellegrino F, Stabile A, Sorce G, Quarta L, Robesti D, Cannoletta D, et al. Added value of prostate-specific antigen density in selecting prostate biopsy candidates among men with elevated prostate-specific antigen and PI-RADS ≥3 lesions on multiparametric magnetic resonance imaging of the prostate: a systematic assessment by PI-RADS score. Eur Urol Focus. 2024;10:634–40.
Görtz M, Radtke JP, Hatiboglu G, Schütz V, Tosev G, Güttlein M, et al. The value of prostate-specific antigen density for prostate imaging-reporting and data system 3 lesions on multiparametric magnetic resonance imaging: a strategy to avoid unnecessary prostate biopsies. Eur Urol Focus. 2021;7:325–31.
Washino S, Okochi T, Saito K, Konishi T, Hirai M, Kobayashi Y, et al. Combination of prostate imaging reporting and data system (PI-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int. 2017;119:225–33.
Rodríguez Cabello MA, Méndez Rubio S, Platas Sancho A, Carballido Rodríguez J. Diagnostic evaluation and incorporation of PSA density and the prostate imaging and data reporting system (PIRADS) version 2 classification in risk-nomograms for prostate cancer. World J Urol. 2022;40:2439–50.
Peters M, Eldred-Evans D, Kurver P, Falagario UG, Connor MJ, Shah TT, et al. Predicting the need for biopsy to detect clinically significant prostate cancer in patients with a magnetic resonance imaging–detected prostate imaging reporting and data system/likert ≥3 lesion: development and multinational external validation of the imperial rapid access to prostate imaging and diagnosis risk score. Eur Urol. 2022;82:559–68.
Zhou Z, Liang Z, Zuo Y, Zhou Y, Yan W, Wu X, et al. Development of a nomogram combining multiparametric magnetic resonance imaging and PSA-related parameters to enhance the detection of clinically significant cancer across different region. Prostate. 2022;82:556–65.
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radio. 2012;22:746–57.
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol. 2016;69:16–40.
Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol. 2019;76:340–351.
Sorce G, Stabile A, Lucianò R, Motterle G, Scuderi S, Barletta F, et al. Multiparametric magnetic resonance imaging of the prostate underestimates tumour volume of small visible lesions. BJU Int. 2022;129:201–7.
Pellegrino F, Stabile A, Mazzone E, Sorce G, Barletta F, De Angelis M, et al. Does previous prostate surgery affect multiparametric magnetic resonance imaging accuracy in detecting clinically significant prostate cancer? Results from a single institution series. Prostate. 2022;82:1170–5.
Stabile A, Barletta F, Motterle G, Pellegrino F, Sorce G, Mazzone E, et al. Optimizing prostate-targeted biopsy schemes in men with multiple mpMRI visible lesions: should we target all suspicious areas? Results of a two institution series. Prostate Cancer Prostatic Dis. 2021;24:1137–42.
Barletta F, Stabile A, Mazzone E, Brembilla G, Sorce G, Pellegrino F, et al. How to optimize follow-up in patients with a suspicious multiparametric MRI and a subsequent negative targeted prostate biopsy. Results from a large, single-institution series. Urol Oncol. 2022;40:103.e17–24.
Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol. 2016;69:428–35.
Magi-Galluzzi C, Montironi R, Epstein JI. Contemporary Gleason grading and novel Grade Groups in clinical practice. Vol. 26, Current Opinion in Urology. Lippincott Williams and Wilkins; 2016. p. 488–92.
Hamm CA, Baumgärtner GL, Padhani AR, Froböse KP, Dräger F, Beetz NL, et al. Reduction of false positives using zone-specific prostate-specific antigen density for prostate MRI-based biopsy decision strategies. Eur Radiol. 2024;34:6229–40.
Kuanar S, Cai J, Nakai H, Nagayama H, Takahashi H, LeGout J, et al. Transition-zone PSA-density calculated from MRI deep learning prostate zonal segmentation model for prediction of clinically significant prostate cancer. Abdom Radiol (NY). 2024;49:3722–34.
Gaudiano C, Bianchi L, Corcioni B, Giunchi F, Schiavina R, Ciccarese F, et al. Evaluating the performance of clinical and radiological data in predicting prostate cancer in prostate imaging reporting and data system version 2.1 category 3 lesions of the peripheral and the transition zones. Int Urol Nephrol. 2022;54:263–71.
Chen Y, Ruan M, Zhou B, Hu X, Wang H, Liu H, et al. Cutoff Values of Prostate Imaging Reporting and Data System Version 2.1 Score in Men With Prostate-specific Antigen Level 4 to 10 ng/mL: Importance of Lesion Location. Clin Genitourin Cancer. 2021;19:288–95.
Mjaess G, Haddad L, Jabbour T, Baudewyns A, Bourgeno HA, Lefebvre Y, et al. Refining clinically relevant cut-offs of prostate specific antigen density for risk stratification in patients with PI-RADS 3 lesions. Prostate Cancer Prostatic Dis. 2025;28:173–179.
Author information
Authors and Affiliations
Contributions
AS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design: LQ, AS Acquisition of data: FP, PS, ML, DC, PZ, AS, AV, FB, SS, RL, AP, EM, LN Analysis and interpretation of data: AS, LQ Drafting of the manuscript: LQ, AS Critical revision of the manuscript for important intellectual content: GB, FDC, RJK, MR, FM, GG, AB Statistical analysis: AS, LQ Supervision: GB, FDC, RJK, MR, FM, GG, AB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of IRCCS San Raffaele Hospital, Milan, Italy. Because this is a retrospective study, there is no approval number for each study. Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quarta, L., Stabile, A., Pellegrino, F. et al. Tailored use of PSA density according to multiparametric MRI index lesion location: results of a large, multi-institutional series. Prostate Cancer Prostatic Dis 29, 111–115 (2026). https://doi.org/10.1038/s41391-025-00987-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41391-025-00987-4