Abstract
Background
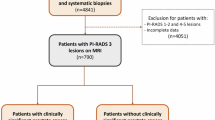
The combination of multiparametric magnetic resonance imaging (MP-MRI) and ultrasound-guided fusion biopsy is increasingly recognized as a valuable tool for diagnosing prostate cancer. However, up to 80% of PI-RADS 3 lesions and 50% of PI-RADS 4 lesions are benign. This study evaluates whether lesion echogenicity observed during MRI-ultrasound fusion biopsy is associated with detecting clinically significant prostate cancer (csPCa).
Methods
In this retrospective analysis (March 2017–February 2022), we reviewed patients who underwent both standard 12-core random biopsies and targeted MP-MRI/US fusion-guided biopsies at our institution. Lesions were categorized as strongly hypoechoic, weakly hypoechoic, or non-hypoechoic based on ultrasound echogenicity. CsPCa was defined as a Gleason score ≥7.
Results
Among 222 biopsy patients, 59.3% were diagnosed with PCa, and 68% had csPCa. Of 420 lesions, 19.1% were strongly hypoechoic (45% csPCa), 29.5% were weakly hypoechoic (25% csPCa), and 51.4% were non-hypoechoic (11.8% csPCa) (p < 0.001). Echogenicity improved csPCa detection for PI-RADS ≤ 3 lesions from 7.5% (non-hypoechoic) to 27.5% (strongly hypoechoic), for PI-RADS 4 from 13.1% to 35.1%, and for PI-RADS 5 from 42% to 63.5%. The ROC analysis demonstrated AUCs of 0.6958 for PI-RADS, 0.6929 for echogenicity, and 0.7434 for their combination (all p < 0.001).
Conclusion
Lesion echogenicity observed during MRI-ultrasound fusion biopsy enhances csPCa detection and complements PI-RADS scoring. Incorporating echogenicity into risk assessment may improve biopsy decision-making and diagnostic accuracy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
SEER [Internet]. [cited 2022 Dec 17]. Cancer of the Prostate—Cancer Stat Facts. Available from: https://seer.cancer.gov/statfacts/html/prost.html.
Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77:38–52.
Ahmed HU, Bosaily AES, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Barr RG, Cosgrove D, Brock M, Cantisani V, Correas JM, Postema AW, et al. WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: Part 5. prostate. Ultrasound Med Biol. 2017;43:27–48.
Boesen L, Nørgaard N, Løgager V, Balslev I, Thomsen HS. Where do transrectal ultrasound- and magnetic resonance imaging-guided biopsies miss significant prostate cancer?. Urology. 2017;110:154–60.
Klotz L, Chin J, Black PC, Finelli A, Anidjar M, Bladou F, et al. Comparison of multiparametric magnetic resonance imaging-targeted biopsy with systematic transrectal ultrasonography biopsy for biopsy-naive men at risk for prostate cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7:534–42.
Drost FJH, Osses D, Nieboer D, Bangma CH, Steyerberg EW, Roobol MJ, et al. Prostate magnetic resonance imaging, with or without magnetic resonance imaging-targeted biopsy, and systematic biopsy for detecting prostate cancer: a Cochrane systematic review and meta-analysis. Eur Urol. 2020;77:78–94.
Valerio M, Donaldson I, Emberton M, Ehdaie B, Hadaschik BA, Marks LS, et al. Detection of Clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur Urol. 2015;68:8–19.
Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MGM. Magnetic resonance imaging–targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015;68:438–50.
Hugosson J, Månsson M, Wallström J, Axcrona U, Carlsson SV, Egevad L, et al. Prostate cancer screening with PSA and MRI followed by targeted biopsy only. N Engl J Med. 2022;387:2126–37.
Nakano Junqueira VC, Zogbi O, Cologna A, dos Reis RB, Tucci S, Reis LO et al. Is a visible (hypoechoic) lesion at biopsy an independent predictor of prostate cancer outcome? Ultrasound Med Biol. 2012;38:1689–94.
Rusu M, Jahanandish H, Vesal S, Li CX, Bhattacharya I, Venkataraman R, et al. ProCUSNet: prostate cancer detection on b-mode transrectal ultrasound using artificial intelligence for targeting during prostate biopsies. Eur Urol Oncol. 2025;8:477–85.
Kinnaird A, Luger F, Cash H, Ghai S, Urdaneta-Salegui LF, Pavlovich CP, et al. Microultrasonography-guided vs MRI-guided biopsy for prostate cancer diagnosis: the OPTIMUM Randomized Clinical Trial. JAMA. 2025;333:1679–87.
Acknowledgements
An abstract of this study was published as part of three conferences: American Urological Association 2024, Mid-Atlantic American Urological Association 2024, and Society of Urologic Oncology 2023. 1. Society of Urologic Oncology: (https://doi.org/10.1016/j.urolonc.2024.01.215). Urologic Oncology: Seminars and Original Investigations. 24th Annual Meeting of the Society of Urologic Oncology, November 28th to December 1st, 2023, Washington DC, USA. https://suo-abstracts.secure-platform.com/a/gallery?roundId=18. 2. Mid-Atlantic American Urologic Association:https://www.canjurol.com/html/free-articles/2024/31-04S1/CJU_V31I3_04S1_03_Abstracts_Aug_Supplement_2024_MAAUAS.pdf. The Canadian Journal of Urology. 82nd Annual Meeting of the Mid-Atlantic Section of the American Urological Association, September 5th to 7th, 2024, White Sulphur Springs, West Virginia, USA. https://auau.auanet.org/content/MAAUA2024. 3. American Urologic Association: (https://doi.org/10.1097/01.JU.0001008672.83391.ed.05) The 2024 Annual Meeting of the American Urological Association, May 3rd to 6th, San Antonio, Texas, USA. https://www.auajournals.org/toc/juro/211/5S.
Author information
Authors and Affiliations
Contributions
GW: data collection, data analysis, figure preparation, drafting of the original manuscript. AK: data analysis, statistical support, figure editing, writing, and revision of the manuscript. MP: data collection, hypothesis generation, assistance with manuscript drafting. SW: initial conceptualization, data collection, and critical input on study design. AJVB: hypothesis generation, initial conceptualization, critical input on study design. MN: provision of resources, critical review of the manuscript. MMS: overall supervision, provision of resources, hypothesis generation, manuscript editing and review, project administration, and guidance.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. Ethical approval for this study was obtained from the University of Maryland, Baltimore Institutional Review Board (IRB) [Protocol/Reference Number: HP-00065772]. Informed consent was not required because of the retrospective nature of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wegner, G., Khan, A., Panagos, M. et al. Ultrasound echogenicity is complementary to PI-RADS for risk stratification of clinically significant prostate cancer. Prostate Cancer Prostatic Dis (2025). https://doi.org/10.1038/s41391-025-01033-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-025-01033-z