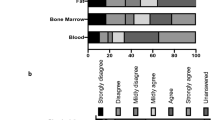
Abstract
Study design
Narrative review by individuals experienced in the recruitment of participants to neurotherapeutic clinical trials in spinal cord injury (SCI).
Objectives
To identify key problems of recruitment and explore potential approaches to overcoming them.
Methods
Published quantitative experience with recruitment of large-scale, experimental neurotherapeutic clinical studies targeting central nervous system and using primary outcome assessments validated for SCI over the last 3 decades was summarized. Based on this experience, potential approaches to improving recruitment were elicited from the authors.
Results
The rate of recruitment has varied between studies, depending on protocol design and other factors, but particularly inclusion/exclusion criteria. The recruitment rate also ranged over an order of magnitude between individual centers in a given study. In older multicenter studies, average recruitment rate was approximately one person per study center per month. More recent trials experienced lower rates of recruitment and potential reasons for this trend were examined. The current roles and potential of various stakeholder organizations in addressing problems of recruitment were explored. In addition, recent developments in methodology may help reduce the number of subjects required for well-powered studies.
Conclusions
Several approaches are emerging to improve clinical trial design, efficacy outcome measures, and quantifiable surrogate markers, all of which should reduce the number of participants required for adequate statistical power. There is a growing sense of cooperation between various stakeholders but more should be done to bring together consumer and provider groups to improve recruitment and the effectiveness and relevance of neurotherapeutic clinical trials.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205.
Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno J, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 2007;45:206–21.
Tuszynski MH, Steeves JD, Fawcett JW, Lammertse D, Kalichman M, Rask C, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial inclusion/exclusion criteria and ethics. Spinal Cord. 2007;45:222–31.
Lammertse D, Tuszynski MH, Steeves JD, Curt A, Fawcett JW, Rask C, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord. 2007;45:232–42.
Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–31.
Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52:110–6.
Lee RS, Noonan VK, Batke J, Ghag A, Paquette SJ, Boyd MC, et al. Feasibility of patient recruitment into clinical trials of experimental treatments for acute spinal cord injury. J Clin Neurosci. 2012;19:1338–43.
Thibault-Halman G, Rivers CS, Bailey CS, Tsai EC, Drew B, Noonan VK, et al. Predicting recruitment feasibility for acute spinal cord injury Clinical Trials in Canada Using National Registry Data. J Neurotrauma. 2017;34:599–606.
Minei JP, Fabian TC, Guffey DM, Newgard CD, Bulger EM, Brasel KJ, et al. Increased trauma center volume is associated with improved survival after severe injury: results of a Resuscitation Outcomes Consortium study. Ann Surg. 2014;260:456–64.
Grossman RG, Toups EG, Frankowski RF, Burau KD, Howley S. North American Clinical Trials Network for the treatment of spinal cord injury: goals and progress. J Neurosurg Spine. 2012;17(1 Suppl):6–10.
Krebs J, Katrin Brust A, Tesini S, Guler M, Mueller G, Velstra I-M, et al. Study participation rate of patients with acute spinal cord injury early during rehabilitation. Spinal Cord. 2015;53:738–42.
Kwon BK, Ghag A, Dvorak MF, Tetzlaff W, Illes J. Expectations of benefit and tolerance to risk of individuals with spinal cord injury regarding potential participation in clinical trials. J Neurotrauma. 2012;29:2727–37.
Dididze M, Green BA, Dietrich WD, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013;51:395–400.
Grossman RG, Fehlings MG, Frankowski RF, Burau KD, Chow DS, Tator C, et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014;31:239–55.
Bracken MB, Shepard MJ, Hellenbrand KG, Collins WF, Leo LS, Freeman DF, et al. Methylprednisolone and neurological function 1 year after spinal cord injury: results of the National Acute Spinal Cord Injury Study. J Neurosurg. 1985;63:704–13.
Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin D, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the second national acute spinal cord injury study. N Engl J Med. 1990;322:1405–11.
Bracken MB, Shepard MJ, Collins WF, Holford TR, Baskin D, Eisenberg HM, et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. J Neurosurg. 1992;76:23–31.
Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Administration of methylprednisolone for 24 or 48 h or tirilazad mesylate for 48 h in the treatment of acute spinal cord injury: Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Am Med Assoc. 1997;277:1597–604.
Geisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord injury—a randomized, placebo controlled trial with GM-1 ganglioside. N Engl J Med. 1991;324:1829–38.
Geisler FH, Coleman WP, Grieco G, Poonian D, the Sygen® Study Group. Recruitment and early treatment in a multicenter study of acute spinal cord injury. Spine. 2001;26:S58–67.
Geisler FH, Coleman WP, Grieco G, Poonian D, the Sygen® Study Group. Measurements and recovery patterns in a multicenter study of acute spinal cord injury. Spine. 2001;26:S68–S86.
Tadié M, Gaviria M, Mathé J-F, Menthonnex Ph, Loubert G, Lagarrigue J. et al. Early care and treatment with a neuroprotective drug, gacyclidine in patients with acute spinal cord injury. Rachis. 2003;15:363–76.
Jones LAT, Lammertse DP, Charlifue SB, Kirshblum SC, Apple DF, Ragnarsson KT, et al. A phase 2 autologous cellular therapy trial in patients with acute, complete spinal cord injury: pragmatics, recruitment, and demographics. Spinal Cord. 2010;48:798–807.
Nance PW, Bugaresti J, Shellenberger K, Sheremata W, Martinez-Arizala A. Efficacy and safety of tizanidine in the treatment of spasticity in patients with spinal cord injury. Neurology. 1994;44(suppl 9):S44–S52.
Cardenas DD, Ditunno J, Graziani V, Lammertse D, Potter P, Sipski M, et al. Phase 2 trial of sustained-release fampridine in chronic spinal cord injury. Spinal Cord. 2007;45:158–68.
Cardenas DD, Ditunno JF, Graziani V, McLain AB, Lammertse DP, Potter PJ, et al. Two phase 3, multicenter, randomized, placebo-controlled clinical trials of fampridine-SR for treatment of spasticity in chronic spinal cord injury. Spinal Cord. 2014;52:70–76.
Fehlings MG, Nakashima H, Nagoshi N, Chow DS, Grossman RG, Kopjar B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): a randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord. 2016;54:8–15.
Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–83.
Illes J, Reimer JC, Kwon BK. Stem cell clinical trials for spinal cord injury: readiness, reluctance, redefinition. Stem Cell Rev. 2011;7:997–1005.
Eijkholt M, Kwon BK, Illes J. Dissociations in the meaning of risk between health-care professionals and individuals with spinal cord injury. Spinal Cord. 2011;51:909–12.
Anderson KD, Cowan RE, Horsewell J. Facilitators and Barriers to Spinal Cord Injury Clinical Trial Participation: Multi-National Perspective of People Living with Spinal Cord Injury. J Neurotrauma. 2016;33:493–9.
Levi AD, Anderson KD, Okondwo DO, Park P, Bryce TN, Kurpad SN, et al. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical SCI. J Neurotrauma. https://doi.org/10.1089/neu.2018.5843.
Furlan JC, Sakakibara BM, Miller WC, Krassioukov AV. Global incidence and prevalence of traumatic spinal cord injury. Can J Neurol Sci. 2013;40:456–64.
Schuld C, Wiese J, Franz S, Putz C, Stierle I, Smoor I, et al. Effect of formal training in scaling, scoring and classification of the International Standards for Neurological Classification of Spinal Cord Injury. Spinal Cord. 2013;51:282–208.
Kucher K, Johns D, Maier D, Abel R, Badke A, Baron H, et al. First-in-man intrathecal application of neurite growth-promoting anti-nogo-a antibodies in acute spinal cord injury. Neurorehabil Neural Repair. 2018;32:578–89.
Edwards P, Arango M, Balica L, Cottingham R, El-Sayed H, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365:1957–9.
Yang ML, Li JJ, So KF, Chen JY, Cheng WS, Wu J, et al. Efficacy and safety of lithium carbonate treatment of chronic spinal cord injuries: a double-blind, randomized, placebo-controlled clinical trial. Spinal Cord. 2012;50:141–6.
Fehlings MG, Nakashima H, Nagoshi N1, Chow DS, Grossman RG, Kopjar B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): a randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord. 2016;54:8–15.
Kwon BK, Hillyer J, Tetzlaff W. Translational research in spinal cord injury: a survey of opinion from the SCI community. J Neurotrauma. 2009;27:21–33.
Kwon BK, Okon EB, Tsai E, Beattie MS, Bresnahan JC, Magnuson DK, et al. A grading system to evaluate objectively the strength of pre-clinical data of acute neuroprotective therapies for clinical translation in spinal cord injury. J Neurotrauma. 2011;28:1525–43.
Kwon BK, Soril LJ, Bacon M, Beattie MS, Blesch A, Bresnahan JC, et al. Demonstrating efficacy in preclinical studies of cellular therapies for spinal cord injury—how much is enough? Exp Neurol. 2013;248:30–44.
Curt A, Levi AD, Schwab JM. Challenges to translation and the hippocratic oath by premature termination of spinal cord stem cell-based trials. JAMA Neurol. 2017;74:635–6.
Wu X, Liu J, Tanadini LG, Lammertse DP, Blight AR, Kramer JL, et al. Challenges for defining minimal clinically important difference (MCID) after spinal cord injury. Spinal Cord. 2015;53:84–91.
Tanadini LG, Steeves JD, Hothorn T, Abel R, Maier D, Schubert M, et al. Identifying homogeneous subgroups in neurological disorders: unbiased recursive partitioning in cervical complete spinal cord injury. Neurorehabil Neural Repair. 2014;28:507–15.
Tanadini LG, Hothorn T, Jones LA, Lammertse DP, Abel R, Maier D, et al. Toward inclusive trial protocols in heterogeneous neurological disorders: prediction-based stratification of participants with incomplete cervical spinal cord injury. Neurorehabil Neural Repair. 2015;29:867–77.
Seibold H, Zeileis A, Hothorn T. Model-based recursive partitioning for subgroup analyses. Int J Biostat. 2016;12:45–63.
Reed R, Mehra M, Kirshblum S, Maier D, Lammertse D, Blight A, et al. Spinal cord ability ruler: an interval scale to measure volitional performance after spinal cord injury. Spinal Cord. 2017;55:730–8.
Itzkovich M, Shefler H, Front L, Gur-Pollack R, Elkayam K, Bluvshtein V, et al. SCIM III (Spinal Cord Independence Measure version III): reliability of assessment by interview and comparison with assessment by observation. Spinal Cord. 2018;56:46–51.
McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomized controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9.
Meurer WJ, Barsan WG. Spinal cord injury neuroprotection and the promise of flexible adaptive clinical trials. World Neurosurg. 2014;82:e541–546.
DasMahapatra P, Raja P, Gilbert J, Wicks P. Clinical trials from the patient perspective: survey in an online patient community. BMC Health Serv Res. 2017;17:166–76.
Funding
Funding was provided by American Spinal Injury Association (ASIA), Christopher & Dana Reeve Foundation, (CDRF) Craig H. Neilsen Foundation (CHN), International Foundation for Research in Paraplegia (IRP), International Spinal Research Trust (ISRT), Rick Hansen Institute (RHI), Spinal Cord Outcomes Partnership Endeavor (SCOPE), and Wings for Life (WfL).
Author information
Authors and Affiliations
Contributions
ARB and JH were responsible for manuscript assembly and also contributed individually to the paper. All remaining authors (AC, JWF, JDG, NK, SNK, BKK, DPL, NW, and JDS) contributed individually to the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Blight, A.R., Hsieh, J., Curt, A. et al. The challenge of recruitment for neurotherapeutic clinical trials in spinal cord injury. Spinal Cord 57, 348–359 (2019). https://doi.org/10.1038/s41393-019-0276-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41393-019-0276-2
This article is cited by
-
Heart rate variability biofeedback in adults with chronic spinal cord injury: a randomised feasibility study
BMC Neurology (2025)
-
Evaluating therapeutic effects of exoskeletons and FES in SCI: integrative review of the literature
Spinal Cord (2025)
-
Challenges and strategies for spinal cord injury research recruitment in rehabilitation hospitals: a single center perspective
Spinal Cord (2025)
-
Ethics and accountability for clinical trials
Spinal Cord (2024)
-
Early and intensive motor training to enhance neurological recovery in people with spinal cord injury: trial protocol
Spinal Cord (2023)