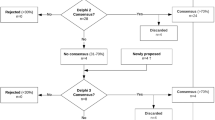
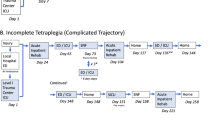
Abstract
Study design
A multisite, randomized, controlled, double-blinded phase I/II clinical trial.
Objective
The purpose of this clinical trial is to evaluate the safety, feasibility and efficacy of pairing noninvasive transcranial direct current stimulation (tDCS) with rehabilitation to promote paretic upper extremity recovery and functional independence in persons living with chronic cervical spinal cord injury (SCI).
Setting
Four-site trial conducted across Cleveland Clinic, Louis Stokes Veterans Affairs Medical Center of Cleveland and MetroHealth Rehabilitation Rehabilitation Institute of Ohio, and Kessler Foundation of New Jersey.
Methods
Forty-four adults (age ≥18 years) with tetraplegia following cervical SCI that occurred ≥1-year ago will participate. Participants will be randomly assigned to receive anodal tDCS or sham tDCS given in combination with upper extremity rehabilitation for 15 sessions each over 3–5 weeks. Assessments will be made twice at baseline separated by at least a 3-week interval, once at end-of-intervention, and once at 3-month follow-up.
Primary outcome measure(s)
Primary outcome measure is upper extremity motor impairment assessed using the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP) scale. Functional abilities will be assessed using Capabilities of Upper Extremity-Test (CUE-T), while functional independence and participation restrictions will be evaluated using the self-care domain of Spinal Cord Independent Measure (SCIM), and Canadian Occupational Performance Measure (COPM).
Secondary outcome measures
Treatment-associated change in corticospinal excitability and output will also be studied using transcranial magnetic stimulation (TMS) and safety (reports of adverse events) and feasibility (attrition, adherence etc.) will also be evaluated.
Trial registration
ClincalTrials.gov identifier NCT03892746. This clinical trial is being performed at four sites within the United States: Cleveland Clinic (lead site), Louis Stokes Cleveland Veterans Affairs Medical Center (VAMC) and MetroHealth Rehabilitation Institute in Ohio, and Kessler Foundation in New Jersey. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. Birmingham. AL: National Spinal Cord injury Statisical Center; 2016.
Simpson LA, Eng JJ, Hsieh JT. Wolfe and the Spinal Cord Injury Rehabilitation Evidence (SCIRE) Research Team DL. The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma. 2012;29:1548–55.
Kloosterman MG, Snoek GJ, Jannink MJ. Systematic review of the effects of exercise therapy on the upper extremity of patients with spinal-cord injury. Spinal Cord. 2009;47:196–203.
Oudega M, Perez MA. Corticospinal reorganization after spinal cord injury. J Physiol. 2012;590:3647–63.
James ND, McMahon SB, Field-Fote EC, Bradbury EJ. Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol. 2018;17:905–17.
Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–23.
Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303.
Potter-Baker KA, Janini DP, Lin YL, Sankarasubramanian V, Cunningham DA, Varnerin NM, et al. Transcranial direct current stimulation (tDCS) paired with massed practice training to promote adaptive plasticity and motor recovery in chronic incomplete tetraplegia: a pilot study. J Spinal Cord Med. 2018;41:503–17.
Cortes M, Medeiros AH, Gandhi A, Lee P, Krebs HI, Thickbroom G, et al. Improved grasp function with transcranial direct current stimulation in chronic spinal cord injury. NeuroRehabilitation. 2017;41:51–9.
Yozbatiran N, Keser Z, Davis M, Stampas A, O’Malley M, Cooper-Hay C, et al. Transcranial direct current stimulation (tDCS) of the primary motor cortex and robot-assisted arm training in chronic incomplete cervical spinal cord injury: A proof of concept sham-randomized clinical study. NeuroRehabilitation. 2016;39:401–11.
Gomes-Osman J, Field-Fote EC. Cortical vs. afferent stimulation as an adjunct to functional task practice training: a randomized, comparative pilot study in people with cervical spinal cord injury. Clin Rehabil. 2015;29:771–82.
Murray LM, Edwards DJ, Ruffini G, Labar D, Stampas A, Pascual-Leone A, et al. Intensity dependent effects of transcranial direct current stimulation on corticospinal excitability in chronic spinal cord injury. Arch Phys Med Rehabil. 2015;96:S114–21.
De Araujo AV, Ribeiro FP, Massetti T, Potter-Baker KA, Cortes M, Plow EB, et al. Effectiveness of anodal transcranial direct current stimulation to improve muscle strength and motor functionality after incomplete spinal cord injury: a systematic review and meta-analysis. Spinal Cord. 2020;58:635–46.
Rupp R, Biering-Sørensen F, Burns SP, Graves DE, Guest J, Jones L, et al. International Standards for Neurological Classification of Spinal Cord Injury: revised 2019. Top Spinal Cord Inj Rehabil. 2021;27:1–22.
Marino RJ, Jones L, Kirshblum S, Tal J, Dasgupta A. Reliability and repeatability of the motor and sensory examination of the international standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2008;31:166–70.
Winstein CJ, Miller JP, Blanton S, Taub E, Uswatte G, Morris D, et al. Methods for a multisite randomized trial to investigate the effect of constraint-induced movement therapy in improving upper extremity function among adults recovering from a cerebrovascular stroke. Neurorehabil Neural Repair. 2003;17:137–52.
Beaulieu LD, Blanchette AK, Mercier C, Bernard-Larocque V, Milot MH. Efficacy, safety, and tolerability of bilateral transcranial direct current stimulation combined to a resistance training program in chronic stroke survivors: a double-blind, randomized, placebo-controlled pilot study. Restor Neurol Neurosci. 2019;37:333–46.
Potter-Baker K, Frederick F, Plow EB. It’s all in your head: driving cortical plasticity to improve muscle contraction below the level of injury. J Spinal Cord Med. 2018;41:592.
Brunoni AR, Schestatsky P, Lotufo PA, Benseñor IM, Fregni F. Comparison of blinding effectiveness between sham tDCS and placebo sertraline in a 6-week major depression randomized clinical trial. Clin Neurophysiol. 2014;125:298–305.
World Health Organization. Towards a common language for functioning, disability, and health: ICF. The international classification of functioning, disability and health. 2002. Retrieved from https://www.who.int/classifications/icf/icfbeginnersguide.pdf on Feb 28, 2022
Kalsi-Ryan S, Curt A, Verrier MC, Fehlings MG. Development of the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP): reviewing measurement specific to the upper limb in tetraplegia. J Neurosurg Spine. 2012;17:65–76.
Marino RJ, Kern SB, Leiby B, Schmidt-Read M, Mulcahey MJ. Reliability and validity of the capabilities of upper extremity test (CUE-T) in subjects with chronic spinal cord injury. J Spinal Cord Med. 2015;38:498–504.
Itzkovich M, Gelernter I, Biering-Sorensen F, Weeks C, Laramee MT, Craven B, et al. The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil Rehabil. 2007;29:1926–33.
Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther. 1990;57:82–7.
Berardi A, Galeoto G, Guarino D, Marquez MA, De Santis R, Valente D, et al. Construct validity, test-retest reliability, and the ability to detect change of the Canadian Occupational Performance Measure in a spinal cord injury population. Spinal Cord Ser Cases. 2019;5:1–8.
Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clin Neurophysiol. 2015;126:1071–107.
Carson RG, Nelson BD, Buick AR, Carroll TJ, Kennedy NC, Mac Cann R. Characterizing changes in the excitability of corticospinal projections to proximal muscles of the upper limb. Brain Stimul. 2013;6:760–8.
Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–38.
Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation evidence from epidural recordings. Exp Brain Res. 1999;128:539–42.
Brasil-Neto JP, McShane LM, Fuhr P, Hallett M, Cohen LG. Topographic mapping of the human motor cortex with magnetic stimulation: factors affecting accuracy and reproducibility. Electroencephalogr Clin Neurophysiol. 1992;85:9–16.
Potter-Baker KA, Janini DP, Frost FS, Chabra P, Varnerin N, Cunningham DA, et al. Reliability of TMS metrics in patients with chronic incomplete spinal cord injury. Spinal Cord. 2016;54:980–90.
Acknowledgements
The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. The authors would like to acknowledge the roles of all team members across study sites, including coordinators, therapists, research assistants, and engineers.
Funding
Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense or U.S. Army. This work was supported by The Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense through the Spinal Cord Injury Research Program under Award No. W81XWH1810530. Here we report on version 1.0 of the protocol approved by the Cleveland Clinic Institutional Review Board (IRB), local site IRBs and the Department of Defense (DoD) Human Research Protection Office (HRPO).
Author information
Authors and Affiliations
Contributions
EPB, KO, KPB, SK, KK, GFF, AB, XW, MAR, SP, and FF were involved in generation of the idea and designing of the protocol. TA, EP, KO, KPB, SK, GFF, MKH, ML, KP, SP, and FB have been involved in protocol revisions and implementation. TA drafted the paper. All authors have been involved in revising and approving the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study has received ethical clearance from IRB of each participating site and the HRPO of the U.S Army Medical Research and Development Command. All participants provide written informed consent before participating. The knowledge from this trial will be disseminated through conference presentations and publications. We aim to target SCI and rehabilitation forums to impact practice, education, research, and policy development.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arora, T., O’Laughlin, K., Potter-Baker, K. et al. Safety and efficacy of transcranial direct current stimulation in upper extremity rehabilitation after tetraplegia: protocol of a multicenter randomized, clinical trial. Spinal Cord 60, 774–778 (2022). https://doi.org/10.1038/s41393-022-00768-z
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41393-022-00768-z