Abstract
Study design
Systematic review with network meta-analysis.
Objective
We explored the efficacy and safety of different drug treatments in patients with spinal-cord injury (SCI)-related neuropathic pain. We investigated which treatment is most suitable for such patients by judging the efficacy and safety of these drugs.
Methods
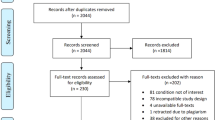
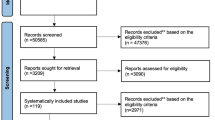
We searched the PubMed, Medline, Embase and Cochrane databases from inception to 31 August 2020. The quality of the included studies was assessed. We selected the proportion of patients whose pain was reduced by ≥50% and the prevalence of adverse effects as the outcome indicators of efficacy and safety, respectively.
Results
We included 15 randomized controlled clinical trials involving five interventions (anticonvulsants, antidepressants, anesthetics, opioids and botulinum toxin A). Based on the proportion of patients with pain reduction ≥50%, the order (from highest to lowest) was anticonvulsants > anesthetics > antidepressants > botulinum toxin A > opioids > placebo. With regard to the prevalence of adverse effects, the order of safety (from highest to lowest) was placebo > antidepressants > botulinum toxin A > anticonvulsants > opioids > anesthetics. Analyzes of efficacy and safety revealed that anticonvulsant, antidepressant and botulinum toxin A have good efficacy and safety.
Conclusion
The efficacy of anticonvulsants, anesthetics, antidepressants, opioids and botulinum toxin A was greater than that of placebo for treatment of SCI-related neuropathic pain. However, the prevalence of adverse effects associated with use of these drugs was also higher than that of placebo. Further analyses based on efficacy and safety revealed anticonvulsants to be more suitable for such patients. In addition, antidepressant and botulinum toxin A may be promising treatments for SCI-related neuropathic pain, however, their effects still need to be further explored due to the small sample size.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Awai L, Bolliger M, Ferguson AR, Courtine G, Curt A. Influence of spinal cord integrity on gait control in human spinal cord injury. Neurorehabil Neural Repair. 2016;30:562–72. https://doi.org/10.1177/1545968315600524
Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–43. https://doi.org/10.1038/nrn1955
Kjell J, Olson L. Rat models of spinal cord injury: from pathology to potential therapies. Dis Model Mech. 2016;9:1125–37. https://doi.org/10.1242/dmm.025833
Demant DT, Lund K, Vollert J, Majer C, Segerdahl M, Finnerup NB, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155:2263–73. https://doi.org/10.1016/j.pain.2014.08.014
Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA, et al. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD003726 https://doi.org/10.1002/14651858.CD003726.pub4
Helfert SM, Reimer M, Hper J, Baron R. Individualized pharmacological treatment of neuropathic pain. Clin Pharmacol Ther. 2015;97:135–42. https://doi.org/10.1038/s41393-020-00595-0
Budh CN, Osteraker AL. Life satisfaction in individuals with a spinal cord injury and pain. Clin Rehabil. 2007;21:89–96. https://doi.org/10.1177/0269215506070313
John DP, Scott JR, Bret LH, Michael JD. Interference due to pain following spinal cord injury: important predictors and impact on quality of life. Pain. 2002;100:231–42. https://doi.org/10.1016/S0304-3959(02)00069-6
Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–57. https://doi.org/10.1016/s0304-3959(02)00452-9
Vranken JH. Mechanisms and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem. 2009;9:71–78. https://doi.org/10.2174/187152409787601932
Finnerup NB, Grydehoj J, Bing J, Johannesen IL, Biering-Sorensen F, Sindrup SH, et al. Levetiracetam in spinal cord injury pain: a randomized controlled trial. Spinal Cord. 2009;47:861–7. https://doi.org/10.1038/sc.2009.55
Finnerup NB, Sindrup SH, Bach FW, Johannesen IL, Jensen TS. Lamotrigine in spinal cord injury pain: a randomized controlled trial. Pain. 2002;96:375–83. https://doi.org/10.1016/s0304-3959(01)00484-5
Levendoglu F, Ogün C, Ozerbil O, Ogün TC, Ugurlu H. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine. 2004;29:743–51. https://doi.org/10.1097/01.brs.0000112068.16108.3a
Norrbrink C, Lundeberg T. Tramadol in neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled trial. Clin J Pain. 2009;25:177–84. https://doi.org/10.1097/AJP.0b013e31818a744
Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006;67:1792–1800. https://doi.org/10.1212/01.wnl.0000244422.45278.ff
Catala-Lopez F, Tobias A, Cameron C, Moher D, Hutton B. Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int. 2014;34:1489–96. https://doi.org/10.1007/s00296-014-2994-2
Hassan S, Ravishankar N, Nair NS. Methodological considerations in network meta-analysis. Int J Med Sci Public Health. 2015;4:588–94. https://doi.org/10.5455/ijmsph.2015.21012015131
Rudroju N, Bansal D, Talakokkula ST, Gudala K, Hota D, Bhansali A, et al. Comparative efficacy and safety of six antidepressants and anticonvulsants in painful diabetic neuropathy: a network meta-analysis. Pain Physician. 2013;16:E705–714.
Cardenas DD, Nieshoff EC, Suda K, Goto S, Sanin L, Knapp LE, et al. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology. 2013;80:533–9. https://doi.org/10.1212/WNL.0b013e318281546b
Drewes AM, Andreasen A, Poulsen LH. Valproate for treatment of chronic central pain after spinal cord injury. A double-blind cross-over study. Paraplegia. 1994;32:565–9. https://doi.org/10.1038/sc.1994.89
Tai Q, Kirshblum S, Chen B, Millis S, Johnston M, DeLisa JA. Gabapentin in the treatment of neuropathic pain after spinal cord injury: a prospective, randomized, double-blind, crossover trial. J Spinal Cord Med. 2002;25:100–5. https://doi.org/10.1080/10790268.2002.11753609
Finnerup NB, Biering-Sorensen F, Johannesen IL, Terkelsen AJ, Juhl GI, Kristensen AD, et al. Intravenous lidocaine relieves spinal cord injury pain: a randomized controlled trial. Anesthesiology. 2005;102:1023–30. https://doi.org/10.1097/00000542-200505000-00023
Kvarnstrom A, Karlsten R, Quiding H, Gordh T. The analgesic effect of intravenous ketamine and lidocaine on pain after spinal cord injury. Acta Anaesthesiol Scand. 2004;48:498–506. https://doi.org/10.1111/j.1399-6576.2003.00330.x
Loubser PG, Donovan WH. Diagnostic spinal anaesthesia in chronic spinal cord injury pain. Paraplegia. 1991;29:25–36. https://doi.org/10.1038/sc.1991.4
Cardenas DD, Warns CA, Turner JA, Marshall H, Brooke MM, Loeser JD, et al. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain. 2002;96:365–73. https://doi.org/10.1016/s0304-3959(01)00483-3
Richards JS, Bombardier CH, Wilson CS, Chiodo AE, Brooks L, Tate DG, et al. Efficacy of venlafaxine XR for the treatment of pain in patients with spinal cord injury and major depression: a randomized, controlled trial. Arch Phys Med Rehabil. 2015;96:680–9. https://doi.org/10.1016/j.apmr.2014.11.024
Siddall PJ, Molloy AR, Walker S, Mather LE, Rutkowski SB, Cousins MJ. The efficacy of intrathecal morphine and clonidine in the treatment of pain after spinal cord injury. Anesth Analg. 2000;91:1493–8. https://doi.org/10.1097/00000539-200012000-00037
Han ZA, Song DH, Oh HM, Chung ME. Botulinum toxin type A for neuropathic pain in patients with spinal cord injury. Ann Neurol. 2016;79:569–78. https://doi.org/10.1002/ana.24605
Bryce TN, Biering-Sorensen F, Finnerup NB, Cardenas DD, Defrin R, Lundeberg T, et al. International spinal cord injury pain classification: part I. Background and description. Spinal Cord. 2012;50:413–7. https://doi.org/10.1038/sc.2011.156. March 6-7, 2009.
Hagen EM, Rekand T. Management of neuropathic pain associated with spinal cord injury. Pain Ther. 2015;4:51–65. https://doi.org/10.1007/s40122-015-0033-y
Moulin D, Boulanger A, Clark AJ, Clarke H, Dao T, Finley GA, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19:328–35. https://doi.org/10.1155/2014/754693
Tan T, Barry P, Reken S, Baker M, Guideline Development G. Pharmacological management of neuropathic pain in non-specialist settings: summary of NICE guidance. BMJ. 2010;340:c1079. https://doi.org/10.1136/bmj.c1079
Mehta S, Mcintyre A, Janzen S, Loh E, Teasell R. A systematic review of pharmacological treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97:1381–91. https://doi.org/10.1016/j.apmr.2015.12.023
Chun A, Levy I, Yang A, Delgado A, Tsai CY, Leung E, et al. Treatment of at-level spinal cord injury pain with botulinum toxin A. Spinal Cord Ser Cases. 2019;5:77 https://doi.org/10.1038/s41394-019-0221-9
Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009;22:467–74. https://doi.org/10.1097/WCO.0b013e3283311e13
Author information
Authors and Affiliations
Contributions
CT performed the study subject and design, data extraction, statistical analysis, interpretation of data and manuscript drafting. LM contributed to the study design, data extraction, statistical analysis and manuscript revising. MF contributed to the study design, data extraction, statistical analysis and interpretation of data. ZZ and FM performed study design, statistical analysis, and critical revision of manuscript. WQ and LX extracted the data. SD and HQ was involved in critical revision of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mei, L., Fengqun, M., Zhengyao, Z. et al. Efficacy and safety of different drug treatments in patients with spinal-cord injury-related neuropathic pain: a network meta-analysis. Spinal Cord 60, 943–953 (2022). https://doi.org/10.1038/s41393-022-00804-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41393-022-00804-y
This article is cited by
-
Spinal cord tethering and syringomyelia after trauma: impact of age and surgical outcome
Scientific Reports (2023)