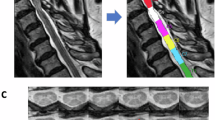
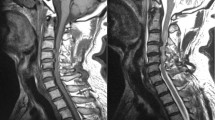
Abstract
Study design
Cross-sectional study.
Objectives
This study’s goal is to report whether Magnetization Transfer Ratio (MTR) can evaluate the severity of white matter (WM) injury in degenerative cervical myelopathy (DCM).
Setting
Laureate Institute of Brain Research, USA; Department of Neurosurgery, University of Oklahoma Health Sciences Center, USA.
Methods
27 DCM patients were aged-matched with 20 healthy controls (HC) and categorized into treatment groups based on modified Japanese Orthopedic Association (mJOA) severity (11 mild and 16 moderate/severe). Regional and tract MTRs were extracted from the two vertebral levels containing maximum compression within magnetization transfer images. MTR differences between groups were assessed using a one-way ANOVA or Kruskal-Wallis test. The association between MTR and mJOA measures was evaluated using Spearman’s correlation.
Results
Significant decreases in MTR were found between HC and moderate/severe groups in the overall (p = 0.0065) and ventral (p = 0.0009) WM regions; and ventral corticospinal (p = 0.0101), ventral reticulospinal (p = 0.0084), spinal lemniscus (p = 0.0079), and fasciculus cuneatus (p = 0.0219) tracts. The spinal lemniscus MTR also significantly decreased between HC and mild groups (p = 0.038). Ventral reticulospinal tract MTR correlated with upper (r = 0.439; p = 0.022) and lower (r = 0.386; p = 0.047) limb motor mJOA scores.
Conclusions
Significant tract-based MTR changes and correlations align with known DCM symptoms, are demonstrated to be lost at the regional level, and display the inhomogeneous compressive damage occurring within DCM spinal cords.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Due to the ongoing nature of data analysis, the datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Code availability
Due to the ongoing nature of the analysis of the patient datasets, the code for this analysis is not yet publicly available. However, until such time that the code used for this analysis is made publicly available on GitHub, it is available upon reasonable request.
References
Hejrati N, Pedro K, Alvi MA, Quddusi A, Fehlings MG. Degenerative cervical myelopathy: where have we been? Where are we now? Where are we going? Acta Neurochir. 2023;165:1105–19.
Lannon M, Kachur E. Degenerative cervical myelopathy: clinical presentation, assessment, and natural history. J Clin Med. 2021;10:3626.
Tetreault L, Kopjar B, Nouri A, Arnold P, Barbagallo G, Bartels R, et al. The modified Japanese Orthopaedic Association scale: establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur Spine J. 2017;26:78–84.
Fehlings MG, Wilson JR, Kopjar B, Yoon ST, Arnold PM, Massicotte EM, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Jt Surg Am. 2013;95:1651–8.
Fehlings MG, Ibrahim A, Tetreault L, Albanese V, Alvarado M, Arnold P, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. Spine (Phila Pa 1976). 2015;40:1322–8.
Zhang JT, Wang LF, Wang S, Li J, Shen Y. Risk factors for poor outcome of surgery for cervical spondylotic myelopathy. Spinal Cord. 2016;54:1127–31.
Zhang RJ, Shen CL, Zhang JX, Zhang XJ, Dong FL, Tao H, et al. Clinical features and surgical outcomes of cervical spondylotic myelopathy in patients of different ages: a retrospective study. Spinal Cord. 2018;56:7–13.
Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine (Phila Pa 1976). 2000;25:670–6.
Pandita N, Gupta S, Raina P, Srivastava A, Hakak AY, Singh O, et al. Neurological recovery pattern in cervical spondylotic myelopathy after anterior surgery: a prospective study with literature review. Asian Spine J. 2019;13:423–31.
Aung WY, Mar S, Benzinger TL. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med. 2013;5:427–40.
D’Souza MM, Choudhary A, Poonia M, Kumar P, Khushu S. Diffusion tensor MR imaging in spinal cord injury. Injury. 2017;48:880–4.
MacKay AL, Laule C. Magnetic resonance of myelin water: an in vivo marker for myelin. Brain Plast. 2016;2:71–91.
Liu Q, Shao H, Liu C, Liu WV, Saeed A, Zhang Q, et al. Quantitative evaluation of the spinal cord compression in patients with cervical spondylotic myelopathy using synthetic MRI. Front Physiol. 2023;14:1140870.
Gloor M, Andelova M, Gaetano L, Papadopoulou A, Burguet Villena F, Sprenger T, et al. Longitudinal analysis of new multiple sclerosis lesions with magnetization transfer and diffusion tensor imaging. Eur Radiol. 2024;34:1680–91.
Cohen-Adad J, El Mendili MM, Lehericy S, Pradat PF, Blancho S, Rossignol S, et al. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage. 2011;55:1024–33.
Suleiman LI, Weber KA 2nd, Rosenthal BD, Bhatt SA, Savage JW, Hsu WK, et al. High-resolution magnetization transfer MRI in patients with cervical spondylotic myelopathy. J Clin Neurosci. 2018;51:57–61.
Cloney MB, Smith ZA, Weber KA 2nd, Parrish TB. Quantitative magnetization transfer MRI measurements of the anterior spinal cord region are associated with clinical outcomes in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2018;43:675–80.
Hopkins BS, Weber KA 2nd, Cloney MB, Paliwal M, Parrish TB, Smith ZA. Tract-Specific Volume Loss on 3T MRI in Patients With Cervical Spondylotic Myelopathy. Spine (Phila Pa 1976). 2018;43:E1204–E9.
Filimonova E, Abdaev M, Vasilenko I, Kubetskij Y, Prokhorov O, Rzaev J. Evaluation of the structural integrity of different spinal cord tracts with magnetization transfer ratio in degenerative cervical myelopathy. Neuroradiology. 2024;66:839–46.
Paliwal M, Weber KA 2nd, Hopkins BS, Cantrell DR, Hoggarth MA, Elliott JM, et al. Magnetization transfer ratio and morphometrics of the spinal cord associates with surgical recovery in patients with degenerative cervical myelopathy. World Neurosurg. 2020;144:e939–e47.
Muhammad F, Weber II KA, Rohan M, Smith ZA. Patterns of cortical thickness alterations in degenerative cervical myelopathy: associations with dexterity and gait dysfunctions. Brain Commun. 2024; [in press].
Cohen-Adad J, Alonso-Ortiz E, Abramovic M, Arneitz C, Atcheson N, Barlow L, et al. Generic acquisition protocol for quantitative MRI of the spinal cord. Nat Protoc. 2021;16:4611–32.
De Leener B, Levy S, Dupont SM, Fonov VS, Stikov N, Louis Collins D, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 2017;145:24–43.
Gros C, De Leener B, Badji A, Maranzano J, Eden D, Dupont SM, et al. Automatic segmentation of the spinal cord and intramedullary multiple sclerosis lesions with convolutional neural networks. Neuroimage. 2019;184:901–15.
Cohen-Adad J, Levy S, Avants B. Slice-by-slice regularized registration for spinal cord MRI: SliceReg. Toronto, Canada: ISMRM; 2015.
De Leener B, Fonov VS, Collins DL, Callot V, Stikov N, Cohen-Adad J. PAM50: Unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage. 2018;165:170–9.
Levy S, Benhamou M, Naaman C, Rainville P, Callot V, Cohen-Adad J. White matter atlas of the human spinal cord with estimation of partial volume effect. Neuroimage. 2015;119:262–71.
Ito T, Oyanagi K, Takahashi H, Takahashi HE, Ikuta F. Cervical spondylotic myelopathy. Clinicopathologic study on the progression pattern and thin myelinated fibers of the lesions of seven patients examined during complete autopsy. Spine (Phila Pa 1976). 1996;21:827–33.
Hardy, TA. Spinal Cord Anatomy and Localization. Continuum (Minneap Minn). 2021;27:12–29.
Yukawa Y, Kato F, Ito K, Horie Y, Nakashima H, Masaaki M, et al. “Ten second step test” as a new quantifiable parameter of cervical myelopathy. Spine (Phila Pa 1976). 2009;34:82–6.
Omori M, Shibuya S, Nakajima T, Endoh T, Suzuki S, Irie S, et al. Hand dexterity impairment in patients with cervical myelopathy: a new quantitative assessment using a natural prehension movement. Behav Neurol. 2018;2018:5138234.
Doita M, Sakai H, Harada T, Nishida K, Miyamoto H, Kaneko T, et al. Evaluation of impairment of hand function in patients with cervical myelopathy. J Spinal Disord Tech. 2006;19:276–80.
Nagata K, Yoshimura N, Muraki S, Hashizume H, Ishimoto Y, Yamada H, et al. Prevalence of cervical cord compression and its association with physical performance in a population-based cohort in Japan: the Wakayama Spine Study. Spine (Phila Pa 1976). 2012;37:1892–8.
Haddas R, Ju KL, Boah A, Kosztowski T, Derman PB. The effect of surgical decompression on functional balance testing in patients with cervical spondylotic myelopathy. Clin Spine Surg. 2019;32:369–76.
Muhammad F, Baha A, Haynes G, Shakir H, Omini M, Martin M, et al. Isolating neurologic deficits in cervical spondylotic myelopathy: a case-controlled study, using the NIH toolbox motor battery. Neurol Clin Pract. 2023;13:e200126.
Muhammad F, Weber II KA, Haynes G, Smith L, Parrish TB, Dhaher Y, et al. Quantifying spinal cord white matter damage beyond compression region in degenerative cervical myelopathy: a magnetization transfer ratio prospective study. Neurosurgery. 2024 [in preparation].
Hameed S, Muhammad F, Haynes G, Smith L, Khan AF, Smith ZA. Early neurological changes in aging cervical spine: insights from PROMIS mobility assessment. Geroscience. 2024;46:3123–34.
Ames CP, Blondel B, Scheer JK, Schwab FJ, Le Huec JC, Massicotte EM, et al. Cervical radiographical alignment: comprehensive assessment techniques and potential importance in cervical myelopathy. Spine (Phila Pa 1976). 2013;38:S149–60.
Buell TJ, Buchholz AL, Quinn JC, Shaffrey CI, Smith JS. Importance of sagittal alignment of the cervical spine in the management of degenerative cervical myelopathy. Neurosurg Clin N Am. 2018;29:69–82.
Patwardhan AG, Khayatzadeh S, Havey RM, Voronov LI, Smith ZA, Kalmanson O, et al. Cervical sagittal balance: a biomechanical perspective can help clinical practice. Eur Spine J. 2018;27:25–38.
Yuan W, Zhu Y, Zhu H, Cui C, Pei L, Huang Z. Preoperative cervical sagittal alignment parameters and their impacts on myelopathy in patients with cervical spondylotic myelopathy: a retrospective study. PeerJ. 2017;5:e4027.
Funding
This work was supported by the National Institutes of Health (NIH) NINDS K23:NS091430 and R01:NS129852-01A1 grants.
Author information
Authors and Affiliations
Contributions
Concept/Idea/Design: GH, FM, TP, YD, KAW, ZAS; Data Collection: Data Collection: GH, FM, AFK, SH; Data Analysis: FM, GH; Writing: GH, FM, LD, KAW, ZAS; Resources: HS, MVH, DD, MR, ZAS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haynes, G., Muhammad, F., Weber, K.A. et al. Tract-specific magnetization transfer ratio provides insights into the severity of degenerative cervical myelopathy. Spinal Cord 62, 700–707 (2024). https://doi.org/10.1038/s41393-024-01036-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41393-024-01036-y